Mycophenolic-acid
Mycophenolic acid (MPA) is a fungal natural product. Mycophenolic Acid is an antineoplastic antibiotic derived from various Penicillium fungal species. Mycophenolic acid is an active metabolite of the prodrug mycophenolate mofetil. Mycophenolic acid inhibits inosine monophosphate dehydrogenase (IMPDH), preventing the formation of guanosine monophosphate and synthesis of lymphocyte DNA that results in inhibition of lymphocyte proliferation, antibody production, cellular adhesion, and migration of T and B lymphocytes. Mycophenolic acid also has antibacterial, antifungal, and antiviral activities. In the compartmentalized biosynthesis of MPA, the acyl-coenzyme A (CoA) hydrolase MpaH' located in peroxisomes catalyzes the highly specific hydrolysis of MPA-CoA to produce the final product MPA
General
Type : Natural, Benzofuran
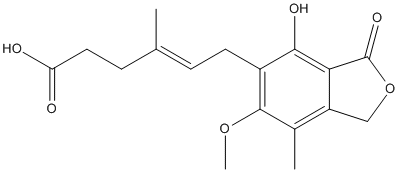
Chemical_Nomenclature : (E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1H-2-benzofuran-5-yl)-4-methylhex-4-enoic acid
Canonical SMILES : CC1=C2COC(=O)C2=C(C(=C1OC)CC=C(C)CCC(=O)O)O
InChI : InChI=1S\/C17H20O6\/c1-9(5-7-13(18)19)4-6-11-15(20)14-12(8-23-17(14)21)10(2)16(11)22-3\/h4,20H,5-8H2,1-3H3,(H,18,19)\/b9-4+
InChIKey : HPNSFSBZBAHARI-RUDMXATFSA-N
Other name(s) : mycophenolic acid || Mycophenolate || Myfortic || Melbex || MPA || MOA || CHEMBL866 || CHEBI:168396 || SCHEMBL4549 || CHEBI:92545
MW : 320.3
Formula : C17H20O6
CAS_number : 24280-93-1
PubChem : 446541
UniChem : HPNSFSBZBAHARI-RUDMXATFSA-N
Target
Families : Mycophenolic-acid ligand of proteins in family
MpaH
Structure :
7DBL
Protein :
penbr-mpaH
References (2)
| Title : Structural basis for substrate specificity of the peroxisomal acyl-CoA hydrolase MpaH' involved in mycophenolic acid biosynthesis - You_2021_FEBS.J_288_5768 |
| Author(s) : You C , Li F , Zhang X , Ma L , Zhang YZ , Zhang W , Li S |
| Ref : Febs J , 288 :5768 , 2021 |
| Abstract : |
| PubMedSearch : You_2021_FEBS.J_288_5768 |
| PubMedID: 33843134 |
| Gene_locus related to this paper: penbr-mpaH |