Phenylalanine
General
Type : Product-like inhibitor, Peptide
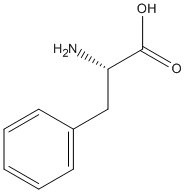
Chemical_Nomenclature : (2S)-2-amino-3-phenylpropanoic acid
Canonical SMILES : C1=CC=C(C=C1)CC(C(=O)O)N
InChI : InChI=1S\/C9H11NO2\/c10-8(9(11)12)6-7-4-2-1-3-5-7\/h1-5,8H,6,10H2,(H,11,12)\/t8-\/m0\/s1
InChIKey : COLNVLDHVKWLRT-QMMMGPOBSA-N
Other name(s) : L-phenylalanine || phenylalanine || CHEBI:58095 || CHEBI:17295 || CHEMBL301523 || (S)-2-Amino-3-phenylpropanoic acid || 3-Phenyl-L-alanine
MW : 165.19
Formula : C9H11NO2
CAS_number : 63-91-2
PubChem : 6140
UniChem : COLNVLDHVKWLRT-QMMMGPOBSA-N
Target
Families : Phenylalanine ligand of proteins in family
Hydrolase_RBBP9_YdeN
Structure :
7OEX
Protein :
human-RBBP9
References (1)
| Title : Mechanism-based traps enable protease and hydrolase substrate discovery - Tang_2022_Nature_602_701 |
| Author(s) : Tang S , Beattie AT , Kafkova L , Petris G , Huguenin-Dezot N , Fiedler M , Freeman M , Chin JW |
| Ref : Nature , 602 :701 , 2022 |
| Abstract : |
| PubMedSearch : Tang_2022_Nature_602_701 |
| PubMedID: 35173328 |
| Gene_locus related to this paper: human-RBBP9 |