Terbutaline-monocarbamate
General
Type : Derivative of Bambuterol, Carbamate, Drug, tert-Butyl, Not A\/B H target, Pro-Drug
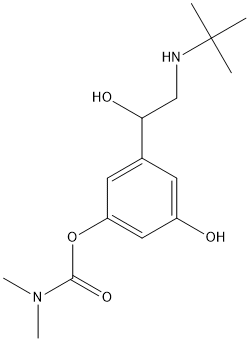
Chemical_Nomenclature : [3-[2-(tert-butylamino)-1-hydroxyethyl]-5-hydroxyphenyl] N,N-dimethylcarbamate
Canonical SMILES : CC(C)(C)NCC(C1=CC(=CC(=C1)OC(=O)N(C)C)O)O
InChI : InChI=1S\/C15H24N2O4\/c1-15(2,3)16-9-13(19)10-6-11(18)8-12(7-10)21-14(20)17(4)5\/h6-8,13,16,18-19H,9H2,1-5H3
InChIKey : ZFYNJGOPBVNUDF-UHFFFAOYSA-N
Other name(s) : D 2439 || D-2439 || Carbamic acid, dimethyl-, 3-(2-((1,1-dimethylethyl)amino)-1-hydroxyethyl)-5-hydroxyphenyl ester || [3-[2-(tert-butylamino)-1-hydroxyethyl]-5-hydroxyphenyl] N,N-dimethylcarbamate || SCHEMBL21674420 || Bambuterol Monocarbamate || [3-[2-(tert-butylamino)-1-hydroxyethyl]-5-hydroxyphenyl] N,N-dimethylcarbamate\;hydrochloride || Bambuterol Monocarbamate Hydrochloride || SCHEMBL11123610
MW : 296.36
Formula : C15H24N2O4
CAS_number : 81732-67-4, 81732-52-7
PubChem : 133750
UniChem : ZFYNJGOPBVNUDF-UHFFFAOYSA-N
Target
References (1)
| Title : Stereoselective inhibition of human butyrylcholinesterase by the enantiomers of bambuterol and their intermediates - Pistolozzi_2015_Drug.Metab.Dispos_43_344 |
| Author(s) : Pistolozzi M , Du H , Wei H , Tan W |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 43 :344 , 2015 |
| Abstract : |
| PubMedSearch : Pistolozzi_2015_Drug.Metab.Dispos_43_344 |
| PubMedID: 25504505 |