Tetracyclic-carbamate-photoswitch-azo
In vitro 10-fold difference in the IC50-values (44.6 nM cis, 424 nM trans). In vivo all or nothing response with complete recovery in a murine cognition-deficit AD model at dosages as low as 0.3 mg/kg
General
Type : Carbamate, Natural_modified, Derivative of Evodiamine, Isoquinoline, Quinazoline, Photoswitch
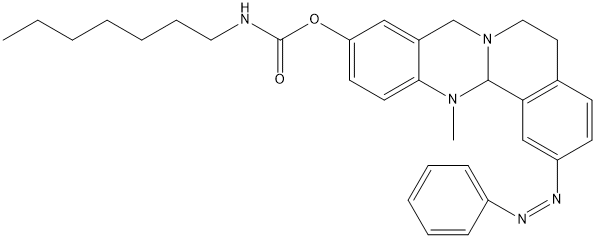
Chemical_Nomenclature : (13-methyl-2-phenyldiazenyl-5,6,8,13a-tetrahydroisoquinolino[1,2-b]quinazolin-10-yl) N-heptylcarbamate
Canonical SMILES : CCCCCCCNC(=O)OC1=CC2=C(C=C1)N(C3C4=C(CCN3C2)C=CC(=C4)N=NC5=CC=CC=C5)C
InChI : InChI=1S\/C31H37N5O2\/c1-3-4-5-6-10-18-32-31(37)38-27-15-16-29-24(20-27)22-36-19-17-23-13-14-26(21-28(23)30(36)35(29)2)34-33-25-11-8-7-9-12-25\/h7-9,11-16,20-21,30H,3-6,10,17-19,22H2,1-2H3,(H,32,37)
InChIKey : YYFAPCBCSCQDFB-UHFFFAOYSA-N
Other name(s) : CHEMBL5220673 || BDBM50606789
MW : 511.7g\/mol
Formula : C31H37N5O2
CAS_number :
PubChem : 164675714
UniChem : YYFAPCBCSCQDFB-UHFFFAOYSA-N
Target
References (1)
| Title : Photoswitchable Pseudoirreversible Butyrylcholinesterase Inhibitors Allow Optical Control of Inhibition in Vitro and Enable Restoration of Cognition in an Alzheimer's Disease Mouse Model upon Irradiation - Scheiner_2022_J.Am.Chem.Soc__ |
| Author(s) : Scheiner M , Sink A , Hoffmann M , Vrigneau C , Endres E , Carles A , Sotriffer C , Maurice T , Decker M |
| Ref : Journal of the American Chemical Society , : , 2022 |
| Abstract : |
| PubMedSearch : Scheiner_2022_J.Am.Chem.Soc__ |
| PubMedID: 35138833 |