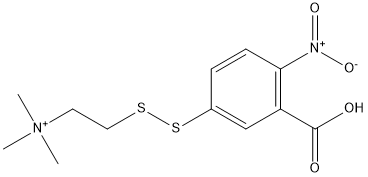
Thiocholine-thionitrobenzoic-acid
The DTNB chemiluminescent probe is not substrate but react with a product of reaction of a substrate hydrolyzed by an enzyme: this compound reacts with thiocholine after hydrolysis of acetylthiocholine by cholinesterases. The end products are Thiocholine-thionitrobenzoic-acid and 2-Nitro-5-sulfanylbenzoic-acid which is yellow colored
General
Type : Trimethylammonium, Ammonium, Sulfur Compound, Benzoate
Chemical_Nomenclature : 2-[(3-carboxy-4-nitrophenyl)disulfanyl]ethyl-trimethylazanium
Canonical SMILES : C[N+](C)(C)CCSSC1=CC(=C(C=C1)[N+](=O)[O-])C(=O)O || C[N+](C)(C)CCSSC1=CC(=C(C=C1)[N+](=O)[O-])C(=O)[O-]
InChI : InChI=1S\/C12H16N2O4S2\/c1-14(2,3)6-7-19-20-9-4-5-11(13(17)18)10(8-9)12(15)16\/h4-5,8H,6-7H2,1-3H3\/p+1 || InChI=1S\/C12H16N2O4S2\/c1-14(2,3)6-7-19-20-9-4-5-11(13(17)18)10(8-9)12(15)16\/h4-5,8H,6-7H2,1-3H3
InChIKey : HRJGWYMNBPLENY-UHFFFAOYSA-O || HRJGWYMNBPLENY-UHFFFAOYSA-N
Other name(s) : SCHEMBL6261498 || 2-nitro-5-[2-(trimethylazaniumyl)ethyldisulfanyl]benzoate || TCh-TNB || A1I9A
MW : 317.4
Formula : C12H17N2O4S2+
CAS_number :
UniChem : HRJGWYMNBPLENY-UHFFFAOYSA-O, HRJGWYMNBPLENY-UHFFFAOYSA-N
Target
Structure :
9QPU
References (2)
| Title : The product of Ellman's reaction inhibits cholinesterases - Stojan_2025_Protein.Sci_34_e70371 |
| Author(s) : Stojan J , Brazzolotto X |
| Ref : Protein Science , 34 :e70371 , 2025 |
| Abstract : |
| PubMedSearch : Stojan_2025_Protein.Sci_34_e70371 |
| PubMedID: 41229096 |
| Gene_locus related to this paper: human-BCHE |
| Title : A new and rapid colorimetric determination of acetylcholinesterase activity - Ellman_1961_Biochem.Pharmacol_7_88 |
| Author(s) : Ellman GL , Courtney KD , Andres V , Featherstone RM , Andres V, Jr. |
| Ref : Biochemical Pharmacology , 7 :88 , 1961 |
| Abstract : |
| PubMedSearch : Ellman_1961_Biochem.Pharmacol_7_88 |
| PubMedID: 13726518 |