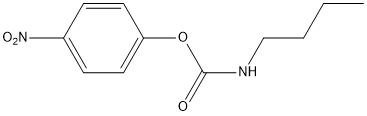
p-nitrophenyl-N-butyl-carbamate
Also inhibits other serine hydrolases trypsine chymotrypsin scofield 1977
General
Chemical_Nomenclature : (4-nitrophenyl) N-butylcarbamate
Canonical SMILES : CCCCNC(=O)OC1=CC=C(C=C1)[N+](=O)[O-]
InChI : InChI=1S\/C11H14N2O4\/c1-2-3-8-12-11(14)17-10-6-4-9(5-7-10)13(15)16\/h4-7H,2-3,8H2,1H3,(H,12,14)
InChIKey : LXLRTTHROGSMBS-UHFFFAOYSA-N
Other name(s) : n-butylcarbamic acid 4-nitrophenyl ester || CHEMBL558048 || 4-Nitrophenyl N-butylcarbamate || 4-Nnbm || CTK5B8633 || DTXSID60979440 || BDBM50295663 || 4-Nitrophenyl hydrogen butylcarbonimidate || Carbamic acid, butyl-, 4-nitrophenyl ester || Carbamic acid,N-butyl-, 4-nitrophenyl ester
MW : 238.24
Formula : C11H14N2O4
CAS_number : 63321-50-6
PubChem : 3017401
UniChem : LXLRTTHROGSMBS-UHFFFAOYSA-N
Target
Families : p-nitrophenyl-N-butyl-carbamate ligand of proteins in family
Cholesterol_esterase
Protein :
pig-i3ltk9
References (2)
| Title : p-Nitrophenyl and cholesteryl-N-alkyl carbamates as inhibitors of cholesterol esterase - Hosie_1987_J.Biol.Chem_262_260 |
| Author(s) : Hosie L , Sutton LD , Quinn DM |
| Ref : Journal of Biological Chemistry , 262 :260 , 1987 |
| Abstract : |
| PubMedSearch : Hosie_1987_J.Biol.Chem_262_260 |
| PubMedID: 3793726 |
| Gene_locus related to this paper: pig-i3ltk9 |
| Title : P-Nitrophenyl carbamates as active-site-specific reagents for serine proteases - |
| Author(s) : Scofield RE , Werner RP , Wold F |
| Ref : Biochemistry , 16 :2492 , 1977 |
| PubMedID: 861216 |