Flupyrazofos
General
Type : Insecticide,Sulfur Compound,Organophosphate,Pyrazole
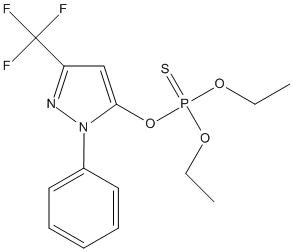
Chemical_Nomenclature : diethoxy-[2-phenyl-5-(trifluoromethyl)pyrazol-3-yl]oxy-sulfanylidene-$l^{5}-phosphane
Canonical SMILES : CCOP(=S)(OCC)OC1=CC(=NN1C2=CC=CC=C2)C(F)(F)F
InChI : InChI=1S\/C14H16F3N2O3PS\/c1-3-20-23(24,21-4-2)22-13-10-12(14(15,16)17)18-19(13)11-8-6-5-7-9-11\/h5-10H,3-4H2,1-2H3
InChIKey : JZUKGAJJLZRHGL-UHFFFAOYSA-N
Other name(s) : DTXSID3058294,LS-109736
MW : 380.32
Formula : C14H16F3N2O3PS
CAS_number : 122431-24-7
PubChem : 11211184
UniChem : JZUKGAJJLZRHGL-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (3)
| Title : Metabolism of flupyrazofos in the isolated perfused rat liver - Jeong_2001_Pest.Manag.Sci_57_427 |
| Author(s) : Jeong CK , Lee H-Y , Kim SB , Choi SJ , Kim J-H , Kim K , Han SS , Lee HS , Lee HY , Kim JH |
| Ref : Pest Manag Sci , 57 :427 , 2001 |
| Abstract : Jeong_2001_Pest.Manag.Sci_57_427 |
| ESTHER : Jeong_2001_Pest.Manag.Sci_57_427 |
| PubMedSearch : Jeong_2001_Pest.Manag.Sci_57_427 |
| PubMedID: 11374159 |
| Title : Aerobic soil metabolism of flupyrazofos - Kim_1998_Pest.Sci_54_237 |
| Author(s) : Kim J-H , Kang K-G , Park C-K , Kim K , Kang B-H , Lee S-K , Roh J-K |
| Ref : Pest Sci , 54 :237 , 1998 |
| Abstract : Kim_1998_Pest.Sci_54_237 |
| ESTHER : Kim_1998_Pest.Sci_54_237 |
| PubMedSearch : Kim_1998_Pest.Sci_54_237 |
| PubMedID: |
| Title : In vitro metabolism of the new insecticide flupyrazofos by rat liver microsomes - Lee_1997_Xenobiotica_27_423 |
| Author(s) : Lee HS , Jeong S , Kim K , Kim JH , Lee SK , Kang BH , Roh JK |
| Ref : Xenobiotica , 27 :423 , 1997 |
| Abstract : Lee_1997_Xenobiotica_27_423 |
| ESTHER : Lee_1997_Xenobiotica_27_423 |
| PubMedSearch : Lee_1997_Xenobiotica_27_423 |
| PubMedID: 9179985 |