Gemigliptin
Gemigliptin (LC15-0444), is an oral anti-hyperglycemic agent (anti-diabetic drug). Currently, gemigliptin has been approved in 11 countries such as India, Columbia, Costa Rica, Panama, and Ecuador, and several clinical studies are in progress in Russia, Mexico, and Thailand.
General
Type : Drug,Piperidine,Pyrimidine,Gliptin,Trifluoro
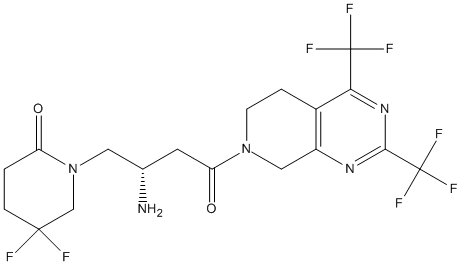
Chemical_Nomenclature : 1-[(2S)-2-amino-4-[2,4-bis(trifluoromethyl)-6,8-dihydro-5H-pyrido[3,4-d]pyrimidin-7-yl]-4-oxobutyl]-5,5-difluoropiperidin-2-one
Canonical SMILES : C1CC(CN(C1=O)CC(CC(=O)N2CCC3=C(C2)N=C(N=C3C(F)(F)F)C(F)(F)F)N)(F)F
InChI : InChI=1S\/C18H19F8N5O2\/c19-16(20)3-1-12(32)31(8-16)6-9(27)5-13(33)30-4-2-10-11(7-30)28-15(18(24,25)26)29-14(10)17(21,22)23\/h9H,1-8,27H2\/t9-\/m0\/s1
InChIKey : ZWPRRQZNBDYKLH-VIFPVBQESA-N
Other name(s) : UNII-5DHU18M5D6,LC15-0444,(S)-1-(2-Amino-4-(2,4-bis(trifluoromethyl)-5,6-dihydropyrido[3,4-d]pyrimidin-7(8H)-yl)-4-oxobutyl)-5,5-difluoropiperidin-2-one,AK170799
MW : 489.37
Formula : C18H19F8N5O2
CAS_number : 911637-19-9
PubChem : 11953153
UniChem : ZWPRRQZNBDYKLH-VIFPVBQESA-N
IUPHAR :
Wikipedia : Gemigliptin
Target
Families : Gemigliptin ligand of proteins in family: DPP4N_Peptidase_S9
Stucture :
Protein : human-DPP4
References (8)
| Title : Gemigliptin, a potent selective dipeptidyl peptidase 4 inhibitor, protects endothelial progenitor cells by oxidative stress via caspase-3 dependent pathway - Lee_2024_Biochem.Biophys.Rep_38_101673 |
| Author(s) : Lee M , Tariq AR , Kim M |
| Ref : Biochem Biophys Rep , 38 :101673 , 2024 |
| Abstract : Lee_2024_Biochem.Biophys.Rep_38_101673 |
| ESTHER : Lee_2024_Biochem.Biophys.Rep_38_101673 |
| PubMedSearch : Lee_2024_Biochem.Biophys.Rep_38_101673 |
| PubMedID: 38444735 |
| Title : Gemigliptin, a DPP4 inhibitor, ameliorates nonalcoholic steatohepatitis through AMP-activated protein kinase-independent and ULK1-mediated autophagy - Song_2023_Mol.Metab__101806 |
| Author(s) : Song Y , Yang H , Kim J , Lee Y , Kim SH , Do IG , Park CY |
| Ref : Mol Metab , :101806 , 2023 |
| Abstract : Song_2023_Mol.Metab__101806 |
| ESTHER : Song_2023_Mol.Metab__101806 |
| PubMedSearch : Song_2023_Mol.Metab__101806 |
| PubMedID: 37739179 |
| Title : Gemigliptin Alleviates Succinate-Induced Hepatic Stellate Cell Activation by Ameliorating Mitochondrial Dysfunction - Nguyen_2022_Endocrinol.Metab.(Seoul)__ |
| Author(s) : Nguyen G , Park SY , Do DV , Choi DH , Cho EH |
| Ref : Endocrinol Metab (Seoul) , : , 2022 |
| Abstract : Nguyen_2022_Endocrinol.Metab.(Seoul)__ |
| ESTHER : Nguyen_2022_Endocrinol.Metab.(Seoul)__ |
| PubMedSearch : Nguyen_2022_Endocrinol.Metab.(Seoul)__ |
| PubMedID: 36377343 |
| Title : Pharmacological profiles of gemigliptin (LC15-0444), a novel dipeptidyl peptidase-4 inhibitor, in vitro and in vivo - Kim_2016_Eur.J.Pharmacol_788_54 |
| Author(s) : Kim SH , Jung E , Yoon MK , Kwon OH , Hwang DM , Kim DW , Kim J , Lee SM , Yim HJ |
| Ref : European Journal of Pharmacology , 788 :54 , 2016 |
| Abstract : Kim_2016_Eur.J.Pharmacol_788_54 |
| ESTHER : Kim_2016_Eur.J.Pharmacol_788_54 |
| PubMedSearch : Kim_2016_Eur.J.Pharmacol_788_54 |
| PubMedID: 27298192 |
| Title : Gemigliptin, a dipeptidyl peptidase-4 inhibitor, inhibits retinal pericyte injury in db\/db mice and retinal neovascularization in mice with ischemic retinopathy - Jung_2015_Biochim.Biophys.Acta_1852_2618 |
| Author(s) : Jung E , Kim J , Kim CS , Kim SH , Cho MH |
| Ref : Biochimica & Biophysica Acta , 1852 :2618 , 2015 |
| Abstract : Jung_2015_Biochim.Biophys.Acta_1852_2618 |
| ESTHER : Jung_2015_Biochim.Biophys.Acta_1852_2618 |
| PubMedSearch : Jung_2015_Biochim.Biophys.Acta_1852_2618 |
| PubMedID: 26391252 |
| Title : Dipeptidyl petidase-IV inhibitor (gemigliptin) inhibits tunicamycin-induced endoplasmic reticulum stress, apoptosis and inflammation in H9c2 cardiomyocytes - Hwang_2014_Mol.Cell.Endocrinol_392_1 |
| Author(s) : Hwang HJ , Jung TW , Ryu JY , Hong HC , Choi HY , Seo JA , Kim SG , Kim NH , Choi KM , Choi DS , Baik SH , Yoo HJ |
| Ref : Mol Cell Endocrinol , 392 :1 , 2014 |
| Abstract : Hwang_2014_Mol.Cell.Endocrinol_392_1 |
| ESTHER : Hwang_2014_Mol.Cell.Endocrinol_392_1 |
| PubMedSearch : Hwang_2014_Mol.Cell.Endocrinol_392_1 |
| PubMedID: 24813659 |
| Title : Pharmacokinetics and pharmacodynamics of LC15-0444, a novel dipeptidyl peptidase IV inhibitor, after multiple dosing in healthy volunteers - Lim_2009_Br.J.Clin.Pharmacol_68_883 |
| Author(s) : Lim KS , Cho JY , Kim BH , Kim JR , Kim HS , Kim DK , Kim SH , Yim HJ , Lee SH , Shin SG , Jang IJ , Yu KS |
| Ref : British Journal of Clinical Pharmacology , 68 :883 , 2009 |
| Abstract : Lim_2009_Br.J.Clin.Pharmacol_68_883 |
| ESTHER : Lim_2009_Br.J.Clin.Pharmacol_68_883 |
| PubMedSearch : Lim_2009_Br.J.Clin.Pharmacol_68_883 |
| PubMedID: 20002082 |
| Title : Pharmacokinetics, pharmacodynamics, and tolerability of the dipeptidyl peptidase IV inhibitor LC15-0444 in healthy Korean men: a dose-block-randomized, double-blind, placebo-controlled, ascending single-dose, Phase I study - Lim_2008_Clin.Ther_30_1817 |
| Author(s) : Lim KS , Kim JR , Choi YJ , Shin KH , Kim KP , Hong JH , Cho JY , Shin HS , Yu KS , Shin SG , Kwon OH , Hwang DM , Kim JA , Jang IJ |
| Ref : Clin Ther , 30 :1817 , 2008 |
| Abstract : Lim_2008_Clin.Ther_30_1817 |
| ESTHER : Lim_2008_Clin.Ther_30_1817 |
| PubMedSearch : Lim_2008_Clin.Ther_30_1817 |
| PubMedID: 19014837 |