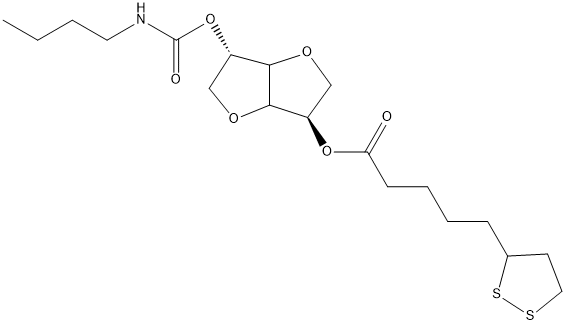
Isosorbide-2-butylcarbamate-5-lipoic-acid-ester
not very specific for BChE IC50 for BChE 170 nM Not as efficient as the benzyl carbamate
General
Type : Isosorbide,Carbamate,Multitarget,Antioxidant
Chemical_Nomenclature : [(2S,3aR,5R,6aR)-5-(butylcarbamoyloxy)-2,3,3a,5,6,6a-hexahydrofuro[3,2-b]furan-2-yl] 5-(dithiolan-3-yl)pentanoate
Canonical SMILES : CCCCNC(=O)OC1CC2C(O1)CC(O2)OC(=O)CCCCC3CCSS3
InChI : InChI=1S\/C19H31NO6S2\/c1-2-3-9-20-19(22)26-18-12-15-14(24-18)11-17(23-15)25-16(21)7-5-4-6-13-8-10-27-28-13\/h13-15,17-18H,2-12H2,1H3,(H,20,22)\/t13?,14-,15-,17+,18-\/m1\/s1
InChIKey : RCFGPZUQMRQWSU-FFDCSCEMSA-N
Other name(s) : Isosorbide-2-butylcarbamate-5-lipoic acid ester,CHEMBL5188267,BDBM50587749,compound 7c
MW : 433.6
Formula : C19H31NO6S2
CAS_number :
PubChem : 168279971
UniChem : RCFGPZUQMRQWSU-FFDCSCEMSA-N
IUPHAR :
Wikipedia :
Target
Families : Isosorbide-2-butylcarbamate-5-lipoic-acid-ester ligand of proteins in family: BCHE
Stucture :
Protein : human-BCHE
References (1)
| Title : Novel Selective Butyrylcholinesterase Inhibitors Incorporating Antioxidant Functionalities as Potential Bimodal Therapeutics for Alzheimer's Disease - Jones_2016_Molecules_21_ |
| Author(s) : Jones M , Wang J , Harmon S , Kling B , Heilmann J , Gilmer JF |
| Ref : Molecules , 21 : , 2016 |
| Abstract : Jones_2016_Molecules_21_ |
| ESTHER : Jones_2016_Molecules_21_ |
| PubMedSearch : Jones_2016_Molecules_21_ |
| PubMedID: 27534722 |