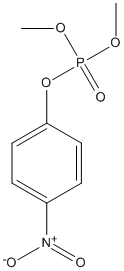
Methylparaoxon
Results from the metabolism in vivo of Methylparathion (oxygen replaces the sulfur atom)
General
Type : Insecticide,Organophosphate,Derivative of Paraoxon,pNP
Chemical_Nomenclature : dimethyl (4-nitrophenyl) phosphate
Canonical SMILES : COP(=O)(OC)OC1=CC=C(C=C1)[N+](=O)[O-]
InChI : InChI=1S\/C8H10NO6P\/c1-13-16(12,14-2)15-8-5-3-7(4-6-8)9(10)11\/h3-6H,1-2H3
InChIKey : BAFQDKPJKOLXFZ-UHFFFAOYSA-N
Other name(s) : Methyl paraoxon,MePOX,Methyl-paraoxon,Paraoxon-methyl,Desmethylnitrophos,Dimethyl paraoxon,Dimethyl O p-nitrophenylphosphate,Dimethyl-p-nitrophenyl phosphate,Dimethyl p-nitrophenyl phosphate,Methyl-E-600,Methyl-E600,BAY 11678,ZINC2040124,CHEMBL26115,SCHEMBL901276,UNII-UE1A2XL95H
MW : 247.14
Formula : C8H10NO6P
CAS_number : 950-35-6
PubChem : 13708
UniChem : BAFQDKPJKOLXFZ-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : Methylparaoxon ligand of proteins in family: ACHE || Carboxypeptidase_S10
Stucture : 3GEL O-methylphosphorylated torpedo acetylcholinesterase obtained by reaction with methyl-parathion (aged)
Protein : torca-ACHE || human-CTSA
References (6)
| Title : Structural and Kinetic Evidence of Aging after Organophosphate Inhibition of Human Cathepsin A - Bouknight_2020_Biochem.Pharmacol__113980 |
| Author(s) : Bouknight KD , Jurkouich KM , Compton JR , Khavrutskii IV , Guelta MA , Harvey SP , Legler PM |
| Ref : Biochemical Pharmacology , :113980 , 2020 |
| Abstract : Bouknight_2020_Biochem.Pharmacol__113980 |
| ESTHER : Bouknight_2020_Biochem.Pharmacol__113980 |
| PubMedSearch : Bouknight_2020_Biochem.Pharmacol__113980 |
| PubMedID: 32305437 |
| Gene_locus related to this paper: human-CTSA |
| Title : Comparison of inhibition kinetics of several organophosphates, including some nerve agent surrogates, using human erythrocyte and rat and mouse brain acetylcholinesterase - Coban_2016_Toxicol.Lett_248_39 |
| Author(s) : Coban A , Carr RL , Chambers HW , Willeford KO , Chambers JE |
| Ref : Toxicol Lett , 248 :39 , 2016 |
| Abstract : Coban_2016_Toxicol.Lett_248_39 |
| ESTHER : Coban_2016_Toxicol.Lett_248_39 |
| PubMedSearch : Coban_2016_Toxicol.Lett_248_39 |
| PubMedID: 26965078 |
| Title : Automated resolution of dichlorvos and methylparaoxon pesticide mixtures employing a Flow Injection system with an inhibition electronic tongue - Valdes-Ramirez_2009_Biosens.Bioelectron_24_1103 |
| Author(s) : Valdes-Ramirez G , Gutierrez M , Del Valle M , Ramirez-Silva MT , Fournier D , Marty JL |
| Ref : Biosensors & Bioelectronics , 24 :1103 , 2009 |
| Abstract : Valdes-Ramirez_2009_Biosens.Bioelectron_24_1103 |
| ESTHER : Valdes-Ramirez_2009_Biosens.Bioelectron_24_1103 |
| PubMedSearch : Valdes-Ramirez_2009_Biosens.Bioelectron_24_1103 |
| PubMedID: 18644713 |
| Title : Acetylcholinesterase-based biosensors for quantification of carbofuran, carbaryl, methylparaoxon, and dichlorvos in 5\% acetonitrile - Valdes-Ramirez_2008_Anal.Bioanal.Chem_392_699 |
| Author(s) : Valdes-Ramirez G , Cortina M , Ramirez-Silva MT , Marty JL |
| Ref : Anal Bioanal Chem , 392 :699 , 2008 |
| Abstract : Valdes-Ramirez_2008_Anal.Bioanal.Chem_392_699 |
| ESTHER : Valdes-Ramirez_2008_Anal.Bioanal.Chem_392_699 |
| PubMedSearch : Valdes-Ramirez_2008_Anal.Bioanal.Chem_392_699 |
| PubMedID: 18663432 |
| Title : Five oximes (K-27, K-48, obidoxime, HI-6 and trimedoxime) in comparison with pralidoxime: survival in rats exposed to methyl-paraoxon - Petroianu_2007_J.Appl.Toxicol_27_453 |
| Author(s) : Petroianu GA , Nurulain SM , Nagelkerke N , Shafiullah M , Kassa J , Kuca K |
| Ref : J Appl Toxicol , 27 :453 , 2007 |
| Abstract : Petroianu_2007_J.Appl.Toxicol_27_453 |
| ESTHER : Petroianu_2007_J.Appl.Toxicol_27_453 |
| PubMedSearch : Petroianu_2007_J.Appl.Toxicol_27_453 |
| PubMedID: 17304644 |
| Title : Interactions of the organophosphates paraoxon and methyl paraoxon with mouse brain acetylcholinesterase - Kardos_2000_Toxicol.Sci_58_118 |
| Author(s) : Kardos SA , Sultatos LG |
| Ref : Toxicol Sci , 58 :118 , 2000 |
| Abstract : Kardos_2000_Toxicol.Sci_58_118 |
| ESTHER : Kardos_2000_Toxicol.Sci_58_118 |
| PubMedSearch : Kardos_2000_Toxicol.Sci_58_118 |
| PubMedID: 11053548 |