S9B
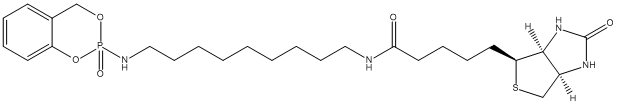
Biotinylated covalent inhibitors of serine esterases allow detection and rapid isolation of neuropathy target esterase (NTE). This enzyme is the primary target site for those organophosphorus esters (OPs) which cause delayed neuropathy. One moiety of S9B (structurally similar to the active metabolite of TOCP) reacts specifically with the catalytic serine residue of NTE; the second moiety is a biotin molecule that allow the NTE-S9B adduct to be captured on avidin-Sepharose.
General
Type : Organophosphate,Cyclic OP,NTE inhibitor,Not A\/B H target,Sulfur Compound,Biotin derivative
Chemical_Nomenclature : 5-[(3aS,4S,6aR)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]-N-[9-[(2-oxo-4H-1,3,2lambda5-benzodioxaphosphinin-2-yl)amino]nonyl]pentanamide
Canonical SMILES : C1C2C(C(S1)CCCCC(=O)NCCCCCCCCCNP3(=O)OCC4=CC=CC=C4O3)NC(=O)N2
InChI : InChI=1S\/C26H41N4O5PS\/c31-24(15-9-8-14-23-25-21(19-37-23)29-26(32)30-25)27-16-10-4-2-1-3-5-11-17-28-36(33)34-18-20-12-6-7-13-22(20)35-36\/h6-7,12-13,21,23,25H,1-5,8-11,14-19H2,(H,27,31)(H,28,33)(H2,29,30,32)\/t21-,23-,25-,36?\/m0\/s1
InChIKey : JZSQBHPKLLZQAH-NYWSWALVSA-N
Other name(s) : 1-(saligenin cyclic phospho)-9-biotinyldiaminononane,DTXSID70935850,N-(9-(4H-1,3,2-Benzodioxaphosphorin-2-ylamino)nonyl)hexahydro-2-oxo-1H-thieno(3,4-d)imidazole-4-pentanamide P-oxide, (3aS-(3aalpha,4beta,6aalpha))-
MW : 552.7
Formula : C26H41N4O5PS
CAS_number : 158040-84-7
PubChem : 133040
UniChem : JZSQBHPKLLZQAH-NYWSWALVSA-N
IUPHAR :
Wikipedia :
Target
Families :
Stucture :
Protein :
References (3)
| Title : Inhibition with spontaneous reactivation and the ongoing inhibition effect of esterases by biotinylated organophosphorus compounds: S9B as a model - Estevez_2010_Chem.Biol.Interact_187_397 |
| Author(s) : Estevez J , Barril J , Vilanova E |
| Ref : Chemico-Biological Interactions , 187 :397 , 2010 |
| Abstract : Estevez_2010_Chem.Biol.Interact_187_397 |
| ESTHER : Estevez_2010_Chem.Biol.Interact_187_397 |
| PubMedSearch : Estevez_2010_Chem.Biol.Interact_187_397 |
| PubMedID: 20493177 |
| Title : Neuropathy target esterase and a homologous Drosophila neurodegeneration-associated mutant protein contain a novel domain conserved from bacteria to man - Lush_1998_Biochem.J_332 ( Pt 1)_1 |
| Author(s) : Lush MJ , Li Y , Read DJ , Willis AC , Glynn P |
| Ref : Biochemical Journal , 332 ( Pt 1) :1 , 1998 |
| Abstract : Lush_1998_Biochem.J_332 ( Pt 1)_1 |
| ESTHER : Lush_1998_Biochem.J_332 ( Pt 1)_1 |
| PubMedSearch : Lush_1998_Biochem.J_332 ( Pt 1)_1 |
| PubMedID: 9576844 |
| Title : Synthesis and characterization of a biotinylated organophosphorus ester for detection and affinity purification of a brain serine esterase: neuropathy target esterase - Glynn_1994_Biochem.J_301 ( Pt 2)_551 |
| Author(s) : Glynn P , Read DJ , Guo R , Wylie S , Johnson MK |
| Ref : Biochemical Journal , 301 ( Pt 2) :551 , 1994 |
| Abstract : Glynn_1994_Biochem.J_301 ( Pt 2)_551 |
| ESTHER : Glynn_1994_Biochem.J_301 ( Pt 2)_551 |
| PubMedSearch : Glynn_1994_Biochem.J_301 ( Pt 2)_551 |
| PubMedID: 8043002 |