ZINC59501712
AChE IC50= 1.35+/-0.08 microM
General
Type : Sulfur Compound,Indole,Sulfonamide,Phthalimido,Benzenesulfonamide
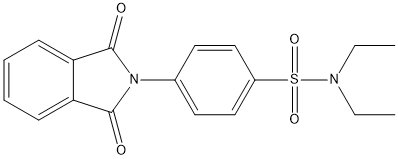
Chemical_Nomenclature : 4-(1,3-dioxoisoindol-2-yl)-N,N-diethylbenzenesulfonamide
Canonical SMILES : CCN(CC)S(=O)(=O)C1=CC=C(C=C1)N2C(=O)C3=CC=CC=C3C2=O
InChI : InChI=1S\/C18H18N2O4S\/c1-3-19(4-2)25(23,24)14-11-9-13(10-12-14)20-17(21)15-7-5-6-8-16(15)18(20)22\/h5-12H,3-4H2,1-2H3
InChIKey : TULQZRPOXBTZLE-UHFFFAOYSA-N
Other name(s) : 4-phthalimidobenzenesulfonamide diethylamine
MW : 358.4
Formula : C18H18N2O4S
CAS_number :
PubChem : 124459204
UniChem : TULQZRPOXBTZLE-UHFFFAOYSA-N
Target
Families : ZINC59501712 ligand of proteins in family
BCHE
Protein :
human-BCHE
References (2)
| Title : 4-Phthalimidobenzenesulfonamide Derivatives as Acetylcholinesterase and Butyrylcholinesterase Inhibitors: DFTs, 3D-QSAR, ADMET, and Molecular Dynamic Simulation - Ejaz_2022_Neurodegener.Dis__ |
| Author(s) : Ejaz SA , Fayyaz A , Mahmood HMK , Aziz M , Ejaz SR , Alkhuriji AF , WA IA-M , Aborode AT , Batiha GE |
| Ref : Neurodegener Dis , : , 2022 |
| Abstract : Ejaz_2022_Neurodegener.Dis__ |
| ESTHER : Ejaz_2022_Neurodegener.Dis__ |
| PubMedSearch : Ejaz_2022_Neurodegener.Dis__ |
| PubMedID: 36288689 |
| Title : Synthesis and molecular docking studies of some 4-phthalimidobenzenesulfonamide derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors - Soyer_2017_J.Enzyme.Inhib.Med.Chem_32_13 |
| Author(s) : Soyer Z , Uysal S , Parlar S , Tarikogullari Dogan AH , Alptuzun V |
| Ref : J Enzyme Inhib Med Chem , 32 :13 , 2017 |
| Abstract : Soyer_2017_J.Enzyme.Inhib.Med.Chem_32_13 |
| ESTHER : Soyer_2017_J.Enzyme.Inhib.Med.Chem_32_13 |
| PubMedSearch : Soyer_2017_J.Enzyme.Inhib.Med.Chem_32_13 |
| PubMedID: 27766908 |