1,5-Seco-vibralactone
Vibralactone is isolated from the basidiomycete fungus Boreostereum vibrans as one of the strongest lipase inhibitors. Its unusual beta-lactone-fused bicycle is derived from an aryl ring moiety via an oxidative ring-expansion prior to an intramolecular cyclization. VibC stehr-Sh.112560 performs the carbocycle formation of an oxepinone to a fused bicyclic beta-lactone
General
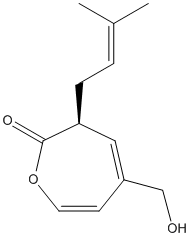
Chemical_Nomenclature : (3S)-5-(hydroxymethyl)-3-(3-methylbut-2-enyl)-3H-oxepin-2-one
Canonical SMILES : CC(=CCC1C=C(C=COC1=O)CO)C
InChI : InChI=1S\/C12H16O3\/c1-9(2)3-4-11-7-10(8-13)5-6-15-12(11)14\/h3,5-7,11,13H,4,8H2,1-2H3\/t11-\/m0\/s1
InChIKey : GBGOKTDQCPDZMS-NSHDSACASA-N
Other name(s) : (1S)-1,5-secovibralactone || 3beta-(3-Methyl-2-butenyl)-5-hydroxymethyloxepin-2(3H)-one
MW : 208.25
Formula : C12H16O3
CAS_number :
PubChem : 102473702
UniChem : GBGOKTDQCPDZMS-NSHDSACASA-N
Target
Structures : No structure
Families : Hormone-sensitive_lipase_like
References (3)
| Title : A Hydrolase-Catalyzed Cyclization Forms the Fused Bicyclic beta-Lactone in Vibralactone - Feng_2020_Angew.Chem.Int.Ed.Engl_59_7209 |
| Author(s) : Feng KN , Yang YL , Xu YX , Zhang Y , Feng T , Huang SX , Liu JK , Zeng Y |
| Ref : Angew Chem Int Ed Engl , 59 :7209 , 2020 |
| Abstract : |
| PubMedSearch : Feng_2020_Angew.Chem.Int.Ed.Engl_59_7209 |
| PubMedID: 32050043 |
| Gene_locus related to this paper: 9agam-VibC , stehr-Sh.112560 |
| Title : A Hydrolase-Catalyzed Cyclization Forms the Fused Bicyclic beta-Lactone in Vibralactone - Feng_2020_Angew.Chem.Int.Ed.Engl_59_7209 |
| Author(s) : Feng KN , Yang YL , Xu YX , Zhang Y , Feng T , Huang SX , Liu JK , Zeng Y |
| Ref : Angew Chem Int Ed Engl , 59 :7209 , 2020 |
| Abstract : |
| PubMedSearch : Feng_2020_Angew.Chem.Int.Ed.Engl_59_7209 |
| PubMedID: 32050043 |
| Gene_locus related to this paper: 9agam-VibC , stehr-Sh.112560 |
| Title : A Hydrolase-Catalyzed Cyclization Forms the Fused Bicyclic beta-Lactone in Vibralactone - Feng_2020_Angew.Chem.Int.Ed.Engl_59_7209 |
| Author(s) : Feng KN , Yang YL , Xu YX , Zhang Y , Feng T , Huang SX , Liu JK , Zeng Y |
| Ref : Angew Chem Int Ed Engl , 59 :7209 , 2020 |
| Abstract : |
| PubMedSearch : Feng_2020_Angew.Chem.Int.Ed.Engl_59_7209 |
| PubMedID: 32050043 |
| Gene_locus related to this paper: 9agam-VibC , stehr-Sh.112560 |