CHEMBL3184534
precursor of Tebuconazole: a chiral triazole fungicide
General
Type : Epoxide, tert-Butyl
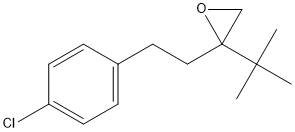
Chemical_Nomenclature : 2-tert-butyl-2-[2-(4-chlorophenyl)ethyl]oxirane
Canonical SMILES : CC(C)(C)C1(CO1)CCC2=CC=C(C=C2)Cl
InChI : InChI=1S\/C14H19ClO\/c1-13(2,3)14(10-16-14)9-8-11-4-6-12(15)7-5-11\/h4-7H,8-10H2,1-3H3
InChIKey : QLCGXXYDHCTVKP-UHFFFAOYSA-N
Other name(s) : 2-(2-(4-Chlorophenyl)ethyl)-2-(1,1-dimethylethyl)oxirane || 2-[2-(4-Chlorophenyl)ethyl]-2-(1,1-dimethylethyl)-oxirane || 2-[2-(4-Chlorophenyl)ethyl]-2-(1,1-dimethyl)-oxirane || 2-(4-chlorophenylethyl)-2-tert.-butyloxirane || CHEMBL454620 || Tebuconazole precusror
MW : 238.75S
Formula : C14H19ClO
CAS_number : 80443-63-6
PubChem : 94684
UniChem : QLCGXXYDHCTVKP-UHFFFAOYSA-N
Target
Structures : No structure
Families : Epoxide_hydrolase
References (3)
| Title : Chemoenzymatic Asymmetric Synthesis of Chiral Triazole Fungicide (R)-Tebuconazole in High Optical Purity Mediated by an Epoxide Hydrolase from Rhodotorula paludigensis - Hu_2024_J.Agric.Food.Chem_72_10428 |
| Author(s) : Hu D , Jia XW , Lu JL , Lu ZY , Tang CD , Xue F , Huang C , Ren QG , He YC |
| Ref : Journal of Agricultural and Food Chemistry , 72 :10428 , 2024 |
| Abstract : |
| PubMedSearch : Hu_2024_J.Agric.Food.Chem_72_10428 |
| PubMedID: 38660720 |
| Gene_locus related to this paper: rhodp-EPH1 |
| Title : Chemoenzymatic Asymmetric Synthesis of Chiral Triazole Fungicide (R)-Tebuconazole in High Optical Purity Mediated by an Epoxide Hydrolase from Rhodotorula paludigensis - Hu_2024_J.Agric.Food.Chem_72_10428 |
| Author(s) : Hu D , Jia XW , Lu JL , Lu ZY , Tang CD , Xue F , Huang C , Ren QG , He YC |
| Ref : Journal of Agricultural and Food Chemistry , 72 :10428 , 2024 |
| Abstract : |
| PubMedSearch : Hu_2024_J.Agric.Food.Chem_72_10428 |
| PubMedID: 38660720 |
| Gene_locus related to this paper: rhodp-EPH1 |
| Title : Chemoenzymatic Asymmetric Synthesis of Chiral Triazole Fungicide (R)-Tebuconazole in High Optical Purity Mediated by an Epoxide Hydrolase from Rhodotorula paludigensis - Hu_2024_J.Agric.Food.Chem_72_10428 |
| Author(s) : Hu D , Jia XW , Lu JL , Lu ZY , Tang CD , Xue F , Huang C , Ren QG , He YC |
| Ref : Journal of Agricultural and Food Chemistry , 72 :10428 , 2024 |
| Abstract : |
| PubMedSearch : Hu_2024_J.Agric.Food.Chem_72_10428 |
| PubMedID: 38660720 |
| Gene_locus related to this paper: rhodp-EPH1 |