Chloramphenicol
Chloramphenicol is an antibiotic first isolated from cultures of Streptomyces venequelae in 1947 but now produced synthetically. It has a relatively simple structure and was the first broad-spectrum antibiotic to be discovered. It acts by interfering with bacterial protein synthesis and is mainly bacteriostatic. EstDL136 reactivated chloramphenicol from its acetyl derivates by counteracting the chloramphenicol acetyltransferase (CAT) activity. It catalyzed the deacetylation of 1- and 3- acetyl and 1,3-diacetyl derivates.EstDL136 also acts as a chloramphenicol hydrolase.It cleaves the amide linkage at C2 of chloramphenicol and thereby confers Cm resistance to bacteria
General
Type : Antibiotic, Drug, pNP, Organochlorine
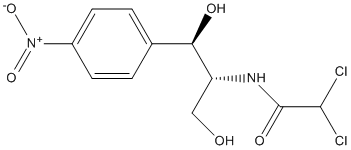
Chemical_Nomenclature : 2,2-dichloro-N-[(1R,2R)-1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl]acetamide
Canonical SMILES : C1=CC(=CC=C1C(C(CO)NC(=O)C(Cl)Cl)O)[N+](=O)[O-]
InChI : InChI=1S\/C11H12Cl2N2O5\/c12-10(13)11(18)14-8(5-16)9(17)6-1-3-7(4-2-6)15(19)20\/h1-4,8-10,16-17H,5H2,(H,14,18)\/t8-,9-\/m1\/s1
InChIKey : WIIZWVCIJKGZOK-RKDXNWHRSA-N
Other name(s) : Chloromycetin || Chlornitromycin || Levomycetin || Chlorocid
MW : 323.12
Formula : C11H12Cl2N2O5
CAS_number : 56-75-7
PubChem : 5959
UniChem : WIIZWVCIJKGZOK-RKDXNWHRSA-N
Target
Structures : 6IEY
Families : Hormone-sensitive_lipase_like
References (15)
| Title : Crystal structure of chloramphenicol-metabolizing enzyme EstDL136 from a metagenome - Kim_2019_PLoS.One_14_e0210298 |
| Author(s) : Kim SH , Kang PA , Han K , Lee SW , Rhee S |
| Ref : PLoS ONE , 14 :e0210298 , 2019 |
| Abstract : |
| PubMedSearch : Kim_2019_PLoS.One_14_e0210298 |
| PubMedID: 30645605 |
| Gene_locus related to this paper: 9bact-g3cr02 |
| Title : Crystal structure of chloramphenicol-metabolizing enzyme EstDL136 from a metagenome - Kim_2019_PLoS.One_14_e0210298 |
| Author(s) : Kim SH , Kang PA , Han K , Lee SW , Rhee S |
| Ref : PLoS ONE , 14 :e0210298 , 2019 |
| Abstract : |
| PubMedSearch : Kim_2019_PLoS.One_14_e0210298 |
| PubMedID: 30645605 |
| Gene_locus related to this paper: 9bact-g3cr02 |
| Title : Crystal structure of chloramphenicol-metabolizing enzyme EstDL136 from a metagenome - Kim_2019_PLoS.One_14_e0210298 |
| Author(s) : Kim SH , Kang PA , Han K , Lee SW , Rhee S |
| Ref : PLoS ONE , 14 :e0210298 , 2019 |
| Abstract : |
| PubMedSearch : Kim_2019_PLoS.One_14_e0210298 |
| PubMedID: 30645605 |
| Gene_locus related to this paper: 9bact-g3cr02 |
| Title : Inactivation of chloramphenicol and florfenicol by a novel chloramphenicol hydrolase - Tao_2012_Appl.Environ.Microbiol_78_6295 |
| Author(s) : Tao W , Lee MH , Wu J , Kim NH , Kim JC , Chung E , Hwang EC , Lee SW |
| Ref : Applied Environmental Microbiology , 78 :6295 , 2012 |
| Abstract : |
| PubMedSearch : Tao_2012_Appl.Environ.Microbiol_78_6295 |
| PubMedID: 22752166 |
| Gene_locus related to this paper: 9bact-g3cr00 , 9bact-g3cr02 |
| Title : Inactivation of chloramphenicol and florfenicol by a novel chloramphenicol hydrolase - Tao_2012_Appl.Environ.Microbiol_78_6295 |
| Author(s) : Tao W , Lee MH , Wu J , Kim NH , Kim JC , Chung E , Hwang EC , Lee SW |
| Ref : Applied Environmental Microbiology , 78 :6295 , 2012 |
| Abstract : |
| PubMedSearch : Tao_2012_Appl.Environ.Microbiol_78_6295 |
| PubMedID: 22752166 |
| Gene_locus related to this paper: 9bact-g3cr00 , 9bact-g3cr02 |
| Title : Inactivation of chloramphenicol and florfenicol by a novel chloramphenicol hydrolase - Tao_2012_Appl.Environ.Microbiol_78_6295 |
| Author(s) : Tao W , Lee MH , Wu J , Kim NH , Kim JC , Chung E , Hwang EC , Lee SW |
| Ref : Applied Environmental Microbiology , 78 :6295 , 2012 |
| Abstract : |
| PubMedSearch : Tao_2012_Appl.Environ.Microbiol_78_6295 |
| PubMedID: 22752166 |
| Gene_locus related to this paper: 9bact-g3cr00 , 9bact-g3cr02 |
| Title : Characterization of two metagenome-derived esterases that reactivate chloramphenicol by counteracting chloramphenicol acetyltransferase - Tao_2011_J.Microbiol.Biotechnol_21_1203 |
| Author(s) : Tao W , Lee MH , Yoon MY , Kim JC , Malhotra S , Wu J , Hwang EC , Lee SW |
| Ref : J Microbiol Biotechnol , 21 :1203 , 2011 |
| Abstract : |
| PubMedSearch : Tao_2011_J.Microbiol.Biotechnol_21_1203 |
| PubMedID: 22210605 |
| Gene_locus related to this paper: 9bact-g3cr00 , 9bact-g3cr02 |
| Title : Characterization of two metagenome-derived esterases that reactivate chloramphenicol by counteracting chloramphenicol acetyltransferase - Tao_2011_J.Microbiol.Biotechnol_21_1203 |
| Author(s) : Tao W , Lee MH , Yoon MY , Kim JC , Malhotra S , Wu J , Hwang EC , Lee SW |
| Ref : J Microbiol Biotechnol , 21 :1203 , 2011 |
| Abstract : |
| PubMedSearch : Tao_2011_J.Microbiol.Biotechnol_21_1203 |
| PubMedID: 22210605 |
| Gene_locus related to this paper: 9bact-g3cr00 , 9bact-g3cr02 |
| Title : Characterization of two metagenome-derived esterases that reactivate chloramphenicol by counteracting chloramphenicol acetyltransferase - Tao_2011_J.Microbiol.Biotechnol_21_1203 |
| Author(s) : Tao W , Lee MH , Yoon MY , Kim JC , Malhotra S , Wu J , Hwang EC , Lee SW |
| Ref : J Microbiol Biotechnol , 21 :1203 , 2011 |
| Abstract : |
| PubMedSearch : Tao_2011_J.Microbiol.Biotechnol_21_1203 |
| PubMedID: 22210605 |
| Gene_locus related to this paper: 9bact-g3cr00 , 9bact-g3cr02 |
| Title : Enzymatic inactivation and reactivation of chloramphenicol by Mycobacterium tuberculosis and Mycobacterium bovis - Sohaskey_2004_FEMS.Microbiol.Lett_240_187 |
| Author(s) : Sohaskey CD |
| Ref : FEMS Microbiology Letters , 240 :187 , 2004 |
| Abstract : |
| PubMedSearch : Sohaskey_2004_FEMS.Microbiol.Lett_240_187 |
| PubMedID: 15522506 |
| Title : Enzymatic inactivation and reactivation of chloramphenicol by Mycobacterium tuberculosis and Mycobacterium bovis - Sohaskey_2004_FEMS.Microbiol.Lett_240_187 |
| Author(s) : Sohaskey CD |
| Ref : FEMS Microbiology Letters , 240 :187 , 2004 |
| Abstract : |
| PubMedSearch : Sohaskey_2004_FEMS.Microbiol.Lett_240_187 |
| PubMedID: 15522506 |
| Title : Enzymatic inactivation and reactivation of chloramphenicol by Mycobacterium tuberculosis and Mycobacterium bovis - Sohaskey_2004_FEMS.Microbiol.Lett_240_187 |
| Author(s) : Sohaskey CD |
| Ref : FEMS Microbiology Letters , 240 :187 , 2004 |
| Abstract : |
| PubMedSearch : Sohaskey_2004_FEMS.Microbiol.Lett_240_187 |
| PubMedID: 15522506 |
| Title : Identification of the chloramphenicol-hydrolyzing enzyme of guinea pig liver as one of the nonspecific carboxylesterases - Kuhn_1982_Biochem.Pharmacol_31_781 |
| Author(s) : Kuhn D , Heymann E |
| Ref : Biochemical Pharmacology , 31 :781 , 1982 |
| Abstract : |
| PubMedSearch : Kuhn_1982_Biochem.Pharmacol_31_781 |
| PubMedID: 7082346 |
| Title : Identification of the chloramphenicol-hydrolyzing enzyme of guinea pig liver as one of the nonspecific carboxylesterases - Kuhn_1982_Biochem.Pharmacol_31_781 |
| Author(s) : Kuhn D , Heymann E |
| Ref : Biochemical Pharmacology , 31 :781 , 1982 |
| Abstract : |
| PubMedSearch : Kuhn_1982_Biochem.Pharmacol_31_781 |
| PubMedID: 7082346 |
| Title : Identification of the chloramphenicol-hydrolyzing enzyme of guinea pig liver as one of the nonspecific carboxylesterases - Kuhn_1982_Biochem.Pharmacol_31_781 |
| Author(s) : Kuhn D , Heymann E |
| Ref : Biochemical Pharmacology , 31 :781 , 1982 |
| Abstract : |
| PubMedSearch : Kuhn_1982_Biochem.Pharmacol_31_781 |
| PubMedID: 7082346 |