Hepoxilin-A3
Hepoxilins are lipid signaling molecules derived from arachidonic acid through the 12-lipoxygenase pathway. Hepoxilins play a role in inflammation, neurotransmission, and formation of skin barrier function. Mammalian hepoxilin hydrolase degrade hepoxilins to trioxilins
General
Type : Natural, Epoxide, Fatty acid
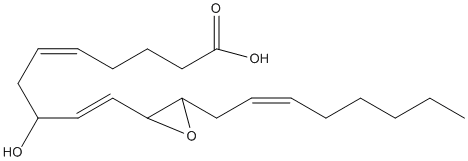
Chemical_Nomenclature : (5E,9E)-8-hydroxy-10-[3-[(E)-oct-2-enyl]oxiran-2-yl]deca-5,9-dienoic acid
Canonical SMILES : CCCCCC=CCC1C(O1)C=CC(CC=CCCCC(=O)O)O
InChI : InChI=1S\/C20H32O4\/c1-2-3-4-5-6-10-13-18-19(24-18)16-15-17(21)12-9-7-8-11-14-20(22)23\/h6-7,9-10,15-19,21H,2-5,8,11-14H2,1H3,(H,22,23)\/b9-7+,10-6+,16-15+ || InChI=1S\/C20H32O4\/c1-2-3-4-5-6-10-13-18-19(24-18)16-15-17(21)12-9-7-8-11-14-20(22)23\/h6-7,9-10,15-19,21H,2-5,8,11-14H2,1H3,(H,22,23)\/b9-7+,10-6+,16-15+\/t17?,18-,19-\/m0\/s1
InChIKey : SGTUOBURCVMACZ-LMIMMGIZSA-N || SGTUOBURCVMACZ-PDSTVSMRSA-N
Other name(s) : Hepoxilin A3 || Hepoxilin A || Hepoxilin || 5,9-Decadienoic acid, 8-hydroxy-10-(3-(2-octenyl)oxiranyl)- || AC1NS4FT || trans-epoxy hydroxy eicosanoids hepoxilin A3 || 8(R,S)-Hydroxy-11S,12S-epoxyeicosa-5Z,9E,14Z-trienoic acid || (5E,9E)-8-hydroxy-10-[(2S,3S)-3-[(E)-oct-2-enyl]oxiran-2-yl]deca-5,9-dienoic acid
MW : 336.47
Formula : C20H32O4
CAS_number : 94161-11-2, 85589-24-8
UniChem : SGTUOBURCVMACZ-LMIMMGIZSA-N, SGTUOBURCVMACZ-PDSTVSMRSA-N
Target
Structures : No structure
Families : Epoxide_hydrolase
References (3)
| Title : Mammalian soluble epoxide hydrolase is identical to liver hepoxilin hydrolase - Cronin_2011_J.Lipid.Res_52_712 |
| Author(s) : Cronin A , Decker M , Arand M |
| Ref : J Lipid Res , 52 :712 , 2011 |
| Abstract : |
| PubMedSearch : Cronin_2011_J.Lipid.Res_52_712 |
| PubMedID: 21217101 |
| Title : Hepoxilin B3 and its enzymatically formed derivative trioxilin B3 are incorporated into phospholipids in psoriatic lesions - Anton_2002_J.Invest.Dermatol_118_139 |
| Author(s) : Anton R , Camacho M , Puig L , Vila L |
| Ref : Journal of Investigative Dermatology , 118 :139 , 2002 |
| Abstract : |
| PubMedSearch : Anton_2002_J.Invest.Dermatol_118_139 |
| PubMedID: 11851887 |
| Title : Hepoxilin A3 is metabolized into its omega-hydroxy metabolite by human neutrophils - |
| Author(s) : Pace-Asciak CR , Reynaud D , Rounova O , Demin P , Pivnitsky KK |
| Ref : Advances in Experimental Medicine & Biology , 469 :535 , 1999 |
| PubMedID: 10667379 |