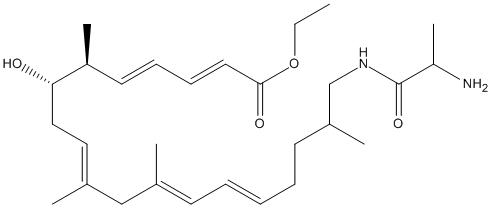
N-alanyl-secovicenilactam-ethyl-ester
Ala captured polyketide intermediate. Elimination of Alanine by VinJ deprotects the compound for action of TE and final cylclisation in the biosynthesis of macrolactam polyketide antibiotics. Analogue of substrate which was used to mimic the elongated polyketide substrate attached to ACP. The compound was docked in the structure 3WMR (Shinohara 2014)
General
Type : Antibiotic, Analogue of substrate
Chemical_Nomenclature : N-alanyl-secovicenilactam-ethyl-ester
Canonical SMILES :
InChI :
InChIKey :
Other name(s) : N-alanyl secovicenilactam ethyl ester
MW : 474.68
Formula : C28H46N2O4
CAS_number :
PubChem :
UniChem :
Target
Structures : No structure
Families : Proline_iminopeptidase
References (6)
| Title : The crystal structure of the amidohydrolase VinJ shows a unique hydrophobic tunnel for its interaction with polyketide substrates - Shinohara_2014_FEBS.Lett_588_995 |
| Author(s) : Shinohara Y , Miyanaga A , Kudo F , Eguchi T |
| Ref : FEBS Letters , 588 :995 , 2014 |
| Abstract : |
| PubMedSearch : Shinohara_2014_FEBS.Lett_588_995 |
| PubMedID: 24530530 |
| Gene_locus related to this paper: strha-q76ky6 |
| Title : The crystal structure of the amidohydrolase VinJ shows a unique hydrophobic tunnel for its interaction with polyketide substrates - Shinohara_2014_FEBS.Lett_588_995 |
| Author(s) : Shinohara Y , Miyanaga A , Kudo F , Eguchi T |
| Ref : FEBS Letters , 588 :995 , 2014 |
| Abstract : |
| PubMedSearch : Shinohara_2014_FEBS.Lett_588_995 |
| PubMedID: 24530530 |
| Gene_locus related to this paper: strha-q76ky6 |
| Title : The crystal structure of the amidohydrolase VinJ shows a unique hydrophobic tunnel for its interaction with polyketide substrates - Shinohara_2014_FEBS.Lett_588_995 |
| Author(s) : Shinohara Y , Miyanaga A , Kudo F , Eguchi T |
| Ref : FEBS Letters , 588 :995 , 2014 |
| Abstract : |
| PubMedSearch : Shinohara_2014_FEBS.Lett_588_995 |
| PubMedID: 24530530 |
| Gene_locus related to this paper: strha-q76ky6 |
| Title : A natural protecting group strategy to carry an amino acid starter unit in the biosynthesis of macrolactam polyketide antibiotics - Shinohara_2011_J.Am.Chem.Soc_133_18134 |
| Author(s) : Shinohara Y , Kudo F , Eguchi T |
| Ref : Journal of the American Chemical Society , 133 :18134 , 2011 |
| Abstract : |
| PubMedSearch : Shinohara_2011_J.Am.Chem.Soc_133_18134 |
| PubMedID: 22010945 |
| Title : A natural protecting group strategy to carry an amino acid starter unit in the biosynthesis of macrolactam polyketide antibiotics - Shinohara_2011_J.Am.Chem.Soc_133_18134 |
| Author(s) : Shinohara Y , Kudo F , Eguchi T |
| Ref : Journal of the American Chemical Society , 133 :18134 , 2011 |
| Abstract : |
| PubMedSearch : Shinohara_2011_J.Am.Chem.Soc_133_18134 |
| PubMedID: 22010945 |
| Title : A natural protecting group strategy to carry an amino acid starter unit in the biosynthesis of macrolactam polyketide antibiotics - Shinohara_2011_J.Am.Chem.Soc_133_18134 |
| Author(s) : Shinohara Y , Kudo F , Eguchi T |
| Ref : Journal of the American Chemical Society , 133 :18134 , 2011 |
| Abstract : |
| PubMedSearch : Shinohara_2011_J.Am.Chem.Soc_133_18134 |
| PubMedID: 22010945 |