W22-methyl-ester
A precursor of Lotrafiban ( a potent non-peptidic antagonist that inhibits platelet aggregation). The S enantiomer is necessary for activity.Hydrolysis of racemic W22-Acetate by Lipase Novozyme 435 results in only the S isomer product
General
Type : Benzodiazepin, Not AlphaBeta Hydrolase target, Drug, Pro-Drug, Azepane
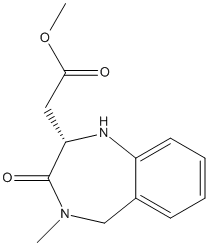
Chemical_Nomenclature : methyl 2-[(2S)-4-methyl-3-oxo-2,5-dihydro-1H-1,4-benzodiazepin-2-yl]acetate
Canonical SMILES : CN1CC2=CC=CC=C2NC(C1=O)CC(=O)OC
InChI : InChI=1S\/C13H16N2O3\/c1-15-8-9-5-3-4-6-10(9)14-11(13(15)17)7-12(16)18-2\/h3-6,11,14H,7-8H2,1-2H3\/t11-\/m0\/s1
InChIKey : OCJURJNFLYOSLL-NSHDSACASA-N
Other name(s) :
MW : 248.27
Formula : C13H16N2O3
CAS_number :
PubChem : 11322612
UniChem : OCJURJNFLYOSLL-NSHDSACASA-N
Target
Structures : No structure
Families : Bacterial_esterase, Canar_LipB
References (6)
| Title : Lipase catalysed resolution of the Lotrafiban intermediate 2,3,4,5-tetrahydro-4-methyl-3-oxo-1 H-1,4-benzodiazepine-2-acetic acid methyl ester in ionic liquids: comparison to the industrial t-butanol process - Roberts_2004_Green.Chem_6_475 |
| Author(s) : Roberts NJ , Seago A , Carey JS , Freer R , Preston C , Lye GJ |
| Ref : Green Chem , 6 :475 , 2004 |
| Abstract : |
| PubMedSearch : Roberts_2004_Green.Chem_6_475 |
| PubMedID: |
| Gene_locus related to this paper: canar-LipB |
| Title : Lipase catalysed resolution of the Lotrafiban intermediate 2,3,4,5-tetrahydro-4-methyl-3-oxo-1 H-1,4-benzodiazepine-2-acetic acid methyl ester in ionic liquids: comparison to the industrial t-butanol process - Roberts_2004_Green.Chem_6_475 |
| Author(s) : Roberts NJ , Seago A , Carey JS , Freer R , Preston C , Lye GJ |
| Ref : Green Chem , 6 :475 , 2004 |
| Abstract : |
| PubMedSearch : Roberts_2004_Green.Chem_6_475 |
| PubMedID: |
| Gene_locus related to this paper: canar-LipB |
| Title : Lipase catalysed resolution of the Lotrafiban intermediate 2,3,4,5-tetrahydro-4-methyl-3-oxo-1 H-1,4-benzodiazepine-2-acetic acid methyl ester in ionic liquids: comparison to the industrial t-butanol process - Roberts_2004_Green.Chem_6_475 |
| Author(s) : Roberts NJ , Seago A , Carey JS , Freer R , Preston C , Lye GJ |
| Ref : Green Chem , 6 :475 , 2004 |
| Abstract : |
| PubMedSearch : Roberts_2004_Green.Chem_6_475 |
| PubMedID: |
| Gene_locus related to this paper: canar-LipB |
| Title : The atomic-resolution structure of a novel bacterial esterase - Bourne_2000_Structure.Fold.Des_8_143 |
| Author(s) : Bourne PC , Isupov MN , Littlechild JA |
| Ref : Structure Fold Des , 8 :143 , 2000 |
| Abstract : |
| PubMedSearch : Bourne_2000_Structure.Fold.Des_8_143 |
| PubMedID: 10673440 |
| Gene_locus related to this paper: alcsp-cxest |
| Title : The atomic-resolution structure of a novel bacterial esterase - Bourne_2000_Structure.Fold.Des_8_143 |
| Author(s) : Bourne PC , Isupov MN , Littlechild JA |
| Ref : Structure Fold Des , 8 :143 , 2000 |
| Abstract : |
| PubMedSearch : Bourne_2000_Structure.Fold.Des_8_143 |
| PubMedID: 10673440 |
| Gene_locus related to this paper: alcsp-cxest |
| Title : The atomic-resolution structure of a novel bacterial esterase - Bourne_2000_Structure.Fold.Des_8_143 |
| Author(s) : Bourne PC , Isupov MN , Littlechild JA |
| Ref : Structure Fold Des , 8 :143 , 2000 |
| Abstract : |
| PubMedSearch : Bourne_2000_Structure.Fold.Des_8_143 |
| PubMedID: 10673440 |
| Gene_locus related to this paper: alcsp-cxest |