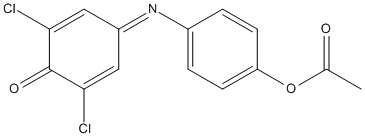
2,6-Dichlorophenolindophenyl-acetate
General
Type : Acetate || Chromogen
Chemical_Nomenclature : [4-[(3,5-dichloro-4-oxocyclohexa-2,5-dien-1-ylidene)amino]phenyl] acetate
Canonical SMILES : CC(=O)OC1=CC=C(C=C1)N=C2C=C(C(=O)C(=C2)Cl)Cl
InChI : InChI=1S\/C14H9Cl2NO3\/c1-8(18)20-11-4-2-9(3-5-11)17-10-6-12(15)14(19)13(16)7-10\/h2-7H,1H3
InChIKey : TTWHMYKNQLOAIV-UHFFFAOYSA-N
Other name(s) : 2,6-Dichlorophenolindophenyl acetate, 2,6-dichloroindophenol acetate, dichloroindophenol acetate, DIPhA, SCHEMBL18605938, DTXSID80885276, NSC90576, ZINC4916805
References (6)
| Title : A Smartphone Camera Colorimetric Assay of Acetylcholinesterase and Butyrylcholinesterase Activity - Pohanka_2021_Sensors.(Basel)_21_ |
| Author(s) : Pohanka M , Zakova J |
| Ref : Sensors (Basel) , 21 : , 2021 |
| Abstract : Pohanka_2021_Sensors.(Basel)_21_ |
| ESTHER : Pohanka_2021_Sensors.(Basel)_21_ |
| PubMedSearch : Pohanka_2021_Sensors.(Basel)_21_ |
| PubMedID: 33807562 |
| Title : Evaluation of 2,6-dichlorophenolindophenol acetate as a substrate for acetylcholinesterase activity assay - Pohanka_2015_J.Enzyme.Inhib.Med.Chem_30_796 |
| Author(s) : Pohanka M , Holas O |
| Ref : J Enzyme Inhib Med Chem , 30 :796 , 2015 |
| Abstract : Pohanka_2015_J.Enzyme.Inhib.Med.Chem_30_796 |
| ESTHER : Pohanka_2015_J.Enzyme.Inhib.Med.Chem_30_796 |
| PubMedSearch : Pohanka_2015_J.Enzyme.Inhib.Med.Chem_30_796 |
| PubMedID: 25672529 |
| Title : Spectrophotometric methods based on 2,6-dichloroindophenol acetate and indoxylacetate for butyrylcholinesterase activity assay in plasma - Pohanka_2013_Talanta_106_281 |
| Author(s) : Pohanka M , Drtinova L |
| Ref : Talanta , 106 :281 , 2013 |
| Abstract : Pohanka_2013_Talanta_106_281 |
| ESTHER : Pohanka_2013_Talanta_106_281 |
| PubMedSearch : Pohanka_2013_Talanta_106_281 |
| PubMedID: 23598128 |
| Title : [How the various substrates activate the process of enzymatic hydrolysis by different cholinesterases] - Basova_2010_Zh.Evol.Biokhim.Fiziol_46_485 |
| Author(s) : Basova NE , Rozengrart EV |
| Ref : Zh Evol Biokhim Fiziol , 46 :485 , 2010 |
| Abstract : Basova_2010_Zh.Evol.Biokhim.Fiziol_46_485 |
| ESTHER : Basova_2010_Zh.Evol.Biokhim.Fiziol_46_485 |
| PubMedSearch : Basova_2010_Zh.Evol.Biokhim.Fiziol_46_485 |
| PubMedID: 21268878 |
| Title : Indophenol Chromogenic Substrates of Cholinesterases of Different Origin - Rozengart_2002_J.Evol.Biochem.Physiol_38_16 |
| Author(s) : Rozengart EV , Basova NE , Suvorov AA , Khovanskikh AE |
| Ref : Journal of Evolutionary Biochemistry and Physiology , 38 :16 , 2002 |
| Abstract : Rozengart_2002_J.Evol.Biochem.Physiol_38_16 |
| ESTHER : Rozengart_2002_J.Evol.Biochem.Physiol_38_16 |
| PubMedSearch : Rozengart_2002_J.Evol.Biochem.Physiol_38_16 |
| PubMedID: |
| Title : [Different aspects of the substrate specificity of the cholinesterase in the optical ganglia of the Pacific Ocean squid Todarodes pacificus] - Rozengart_1996_Zh.Evol.Biokhim.Fiziol_32_384 |
| Author(s) : Rozengart EV , Basova NE , Khovanskikh AE , Epshtein LM |
| Ref : Zhurnal Evoliutsionnoi Biokhimii i Fiziologii , 32 :384 , 1996 |
| Abstract : Rozengart_1996_Zh.Evol.Biokhim.Fiziol_32_384 |
| ESTHER : Rozengart_1996_Zh.Evol.Biokhim.Fiziol_32_384 |
| PubMedSearch : Rozengart_1996_Zh.Evol.Biokhim.Fiziol_32_384 |
| PubMedID: 9054172 |