Ketoprofen-Vinyl-Ester
General
Type : Profen prodrug || Pro-Drug || Drug || Aryl ester || Propionate
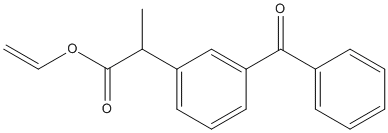
Chemical_Nomenclature : ethenyl 2-(3-benzoylphenyl)propanoate
Canonical SMILES : CC(C1=CC=CC(=C1)C(=O)C2=CC=CC=C2)C(=O)OC=C
InChI : InChI=1S\/C18H16O3\/c1-3-21-18(20)13(2)15-10-7-11-16(12-15)17(19)14-8-5-4-6-9-14\/h3-13H,1H2,2H3
InChIKey : UPFGCMKGQFYQGF-UHFFFAOYSA-N
Other name(s) : Ketoprofen Vinyl Ester
MW : 280.32
Formula : C18H16O3
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Canar_LipB
References (3)
| Title : Immobilization of Aspergillus terreus lipase in self-assembled hollow nanospheres for enantioselective hydrolysis of ketoprofen vinyl ester - Hu_2015_J.Biotechnol_194_12 |
| Author(s) : Hu C , Wang N , Zhang W , Zhang S , Meng Y , Yu X |
| Ref : J Biotechnol , 194 :12 , 2015 |
| Abstract : Hu_2015_J.Biotechnol_194_12 |
| ESTHER : Hu_2015_J.Biotechnol_194_12 |
| PubMedSearch : Hu_2015_J.Biotechnol_194_12 |
| PubMedID: 25483320 |
| Title : Insight into substituent effects in Cal-B catalyzed transesterification by combining experimental and theoretical approaches - Ni_2013_J.Mol.Model_19_349 |
| Author(s) : Ni Z , Lin X |
| Ref : J Mol Model , 19 :349 , 2013 |
| Abstract : Ni_2013_J.Mol.Model_19_349 |
| ESTHER : Ni_2013_J.Mol.Model_19_349 |
| PubMedSearch : Ni_2013_J.Mol.Model_19_349 |
| PubMedID: 22923134 |
| Title : Integrating In Silico and In vitro approaches to dissect the stereoselectivity of Bacillus subtilis lipase A toward ketoprofen vinyl ester - Ni_2011_Chem.Biol.Drug.Des_78_301 |
| Author(s) : Ni Z , Zhou P , Jin X , Lin XF |
| Ref : Chemical Biology Drug Des , 78 :301 , 2011 |
| Abstract : Ni_2011_Chem.Biol.Drug.Des_78_301 |
| ESTHER : Ni_2011_Chem.Biol.Drug.Des_78_301 |
| PubMedSearch : Ni_2011_Chem.Biol.Drug.Des_78_301 |
| PubMedID: 21477088 |
| Gene_locus related to this paper: bacsu-lip |