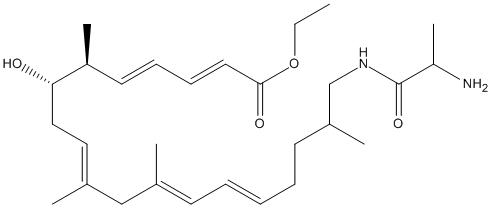
N-alanyl-secovicenilactam-ethyl-ester
General
Type : Antibiotic || Analogue of substrate
Chemical_Nomenclature : N-alanyl-secovicenilactam-ethyl-ester
Canonical SMILES :
InChI :
InChIKey :
Other name(s) : N-alanyl secovicenilactam ethyl ester
MW : 474.68
Formula : C28H46N2O4
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Proline_iminopeptidase
References (2)
| Title : The crystal structure of the amidohydrolase VinJ shows a unique hydrophobic tunnel for its interaction with polyketide substrates - Shinohara_2014_FEBS.Lett_588_995 |
| Author(s) : Shinohara Y , Miyanaga A , Kudo F , Eguchi T |
| Ref : FEBS Letters , 588 :995 , 2014 |
| Abstract : Shinohara_2014_FEBS.Lett_588_995 |
| ESTHER : Shinohara_2014_FEBS.Lett_588_995 |
| PubMedSearch : Shinohara_2014_FEBS.Lett_588_995 |
| PubMedID: 24530530 |
| Gene_locus related to this paper: strha-q76ky6 |
| Title : A natural protecting group strategy to carry an amino acid starter unit in the biosynthesis of macrolactam polyketide antibiotics - Shinohara_2011_J.Am.Chem.Soc_133_18134 |
| Author(s) : Shinohara Y , Kudo F , Eguchi T |
| Ref : Journal of the American Chemical Society , 133 :18134 , 2011 |
| Abstract : Shinohara_2011_J.Am.Chem.Soc_133_18134 |
| ESTHER : Shinohara_2011_J.Am.Chem.Soc_133_18134 |
| PubMedSearch : Shinohara_2011_J.Am.Chem.Soc_133_18134 |
| PubMedID: 22010945 |