1,3-Dibenzylurea
General
Type : Natural, Urea derivative
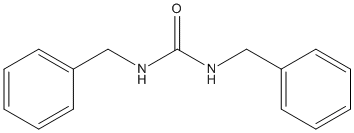
Chemical_Nomenclature : 1,3-dibenzylurea
Canonical SMILES : C1=CC=C(C=C1)CNC(=O)NCC2=CC=CC=C2
InChI : InChI=1S\/C15H16N2O\/c18-15(16-11-13-7-3-1-4-8-13)17-12-14-9-5-2-6-10-14\/h1-10H,11-12H2,(H2,16,17,18)
InChIKey : KATOLVAXCGIBLO-UHFFFAOYSA-N
Other name(s) : N,N'-Dibenzylurea || 1466-67-7 || Sym-Dibenzylurea || Urea, N,N'-bis(phenylmethyl)- || 1,3-dibenzyl urea
Target
Families : 1,3-Dibenzylurea ligand of proteins in family
Epoxide_hydrolase
References (4)
| Title : Occurrence of urea-based soluble epoxide hydrolase inhibitors from the plants in the order Brassicales - Kitamura_2017_PLoS.One_12_e0176571 |
| Author(s) : Kitamura S , Morisseau C , Harris TR , Inceoglu B , Hammock BD |
| Ref : PLoS ONE , 12 :e0176571 , 2017 |
| Abstract : |
| PubMedSearch : Kitamura_2017_PLoS.One_12_e0176571 |
| PubMedID: 28472063 |
| Title : Potent natural soluble epoxide hydrolase inhibitors from Pentadiplandra brazzeana baillon: synthesis, quantification, and measurement of biological activities in vitro and in vivo - Kitamura_2015_PLoS.One_10_e0117438 |
| Author(s) : Kitamura S , Morisseau C , Inceoglu B , Kamita SG , De Nicola GR , Nyegue M , Hammock BD |
| Ref : PLoS ONE , 10 :e0117438 , 2015 |
| Abstract : |
| PubMedSearch : Kitamura_2015_PLoS.One_10_e0117438 |
| PubMedID: 25659109 |
| Title : Rare dipeptide and urea derivatives from roots of Moringa oleifera as potential anti-inflammatory and antinociceptive agents - Sashidhara_2009_Eur.J.Med.Chem_44_432 |
| Author(s) : Sashidhara KV , Rosaiah JN , Tyagi E , Shukla R , Raghubir R , Rajendran SM |
| Ref : Eur Journal of Medicinal Chemistry , 44 :432 , 2009 |
| Abstract : |
| PubMedSearch : Sashidhara_2009_Eur.J.Med.Chem_44_432 |
| PubMedID: 18243423 |
| Title : Urea derivatives from Pentadiplandra brazzeana - Tsopmo_1999_J.Nat.Prod_62_1435 |
| Author(s) : Tsopmo A , Ngnokam D , Ngamga D , Ayafor JF , Sterner O |
| Ref : Journal of Natural Products , 62 :1435 , 1999 |
| Abstract : |
| PubMedSearch : Tsopmo_1999_J.Nat.Prod_62_1435 |
| PubMedID: 10543911 |