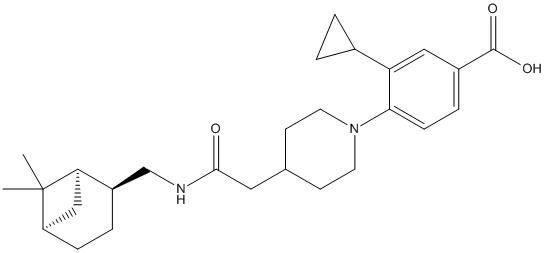
AS2586144
IC50 = 5.9 nM for rat sEH = 16nM for human sEH
General
Type : Piperidine, Benzoate, Cyclopropyl
Chemical_Nomenclature : 3-cyclopropyl-4-{4-[2-({[(1S,2R,5S)-6,6-dimethylbicyclo[3,1,1]heptan-2-yl]methyl}amino)-2-oxoethyl]piperidin-1-yl}benzoic acid monohydrochloride
Canonical SMILES : CC1([C@@H]2CC[C@@H]([C@H]1C2)CNC(=O)CC3CCN(CC3)C4=C(C=C(C=C4)C(=O)O)C5CC5)C
InChI : InChI=1S\/C27H38N2O3\/c1-27(2)21-7-5-20(23(27)15-21)16-28-25(30)13-17-9-11-29(12-10-17)24-8-6-19(26(31)32)14-22(24)18-3-4-18\/h6,8,14,17-18,20-21,23H,3-5,7,9-13,15-16H2,1-2H3,(H,28,30)(H,31,32)\/t20-,21-,23-\/m1\/s1
InChIKey : TZTAAXZTTVCUFV-MQSCRBSSSA-N
Other name(s) :
Target
Families : AS2586144 ligand of proteins in family
Epoxide_hydrolase
Protein :
human-EPHX2
References (6)
| Title : Role of epoxy-fatty acids and epoxide hydrolases in the pathology of neuro-inflammation - Kodani_2019_Biochimie_159_59 |
| Author(s) : Kodani SD , Morisseau C |
| Ref : Biochimie , 159 :59 , 2019 |
| Abstract : |
| PubMedSearch : Kodani_2019_Biochimie_159_59 |
| PubMedID: 30716359 |
| Title : Role of epoxy-fatty acids and epoxide hydrolases in the pathology of neuro-inflammation - Kodani_2019_Biochimie_159_59 |
| Author(s) : Kodani SD , Morisseau C |
| Ref : Biochimie , 159 :59 , 2019 |
| Abstract : |
| PubMedSearch : Kodani_2019_Biochimie_159_59 |
| PubMedID: 30716359 |
| Title : Role of epoxy-fatty acids and epoxide hydrolases in the pathology of neuro-inflammation - Kodani_2019_Biochimie_159_59 |
| Author(s) : Kodani SD , Morisseau C |
| Ref : Biochimie , 159 :59 , 2019 |
| Abstract : |
| PubMedSearch : Kodani_2019_Biochimie_159_59 |
| PubMedID: 30716359 |
| Title : Single administration of soluble epoxide hydrolase inhibitor suppresses neuroinflammation and improves neuronal damage after cardiac arrest in mice - Taguchi_2016_Neurosci.Res_111_56 |
| Author(s) : Taguchi N , Nakayama S , Tanaka M |
| Ref : Neurosci Res , 111 :56 , 2016 |
| Abstract : |
| PubMedSearch : Taguchi_2016_Neurosci.Res_111_56 |
| PubMedID: 27184295 |
| Title : Single administration of soluble epoxide hydrolase inhibitor suppresses neuroinflammation and improves neuronal damage after cardiac arrest in mice - Taguchi_2016_Neurosci.Res_111_56 |
| Author(s) : Taguchi N , Nakayama S , Tanaka M |
| Ref : Neurosci Res , 111 :56 , 2016 |
| Abstract : |
| PubMedSearch : Taguchi_2016_Neurosci.Res_111_56 |
| PubMedID: 27184295 |
| Title : Single administration of soluble epoxide hydrolase inhibitor suppresses neuroinflammation and improves neuronal damage after cardiac arrest in mice - Taguchi_2016_Neurosci.Res_111_56 |
| Author(s) : Taguchi N , Nakayama S , Tanaka M |
| Ref : Neurosci Res , 111 :56 , 2016 |
| Abstract : |
| PubMedSearch : Taguchi_2016_Neurosci.Res_111_56 |
| PubMedID: 27184295 |