CHEMBL4177075
biooxidisable prodrug: human ACHE IC50 0.154 microM || . Electron-withdrawing-group
General
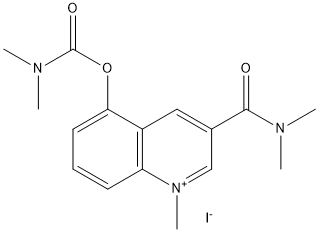
Chemical_Nomenclature : [3-(dimethylcarbamoyl)-1-methylquinolin-1-ium-5-yl] N,N-dimethylcarbamate\;iodide
Canonical SMILES : C[N+]1=CC(=CC2=C1C=CC=C2OC(=O)N(C)C)C(=O)N(C)C.[I-]
InChI : InChI=1S\/C16H20N3O3.HI\/c1-17(2)15(20)11-9-12-13(19(5)10-11)7-6-8-14(12)22-16(21)18(3)4\;\/h6-10H,1-5H3\;1H\/q+1\;\/p-1
InChIKey : KHYLGBRHLIMEHR-UHFFFAOYSA-M
Other name(s) : [3-(Dimethylcarbamoyl)-1-methyl-quinolin-1-ium-5-yl] N,N-dimethylcarbamate iodide || Compound 2A || BDBM50449492
Target
Families : CHEMBL4177075 ligand of proteins in family
ACHE
Protein :
human-ACHE
References (1)
| Title : Rational design of carbamate-based dual binding site and central AChE inhibitors by a 'biooxidisable' prodrug approach: Synthesis, invitro evaluation and docking studies - Tintas_2018_Eur.J.Med.Chem_155_171 |
| Author(s) : Tintas ML , Gembus V , Alix F , Barre A , Coadou G , Truong L , Sebban M , Papamical C , Oulyadi H , Levacher V |
| Ref : Eur Journal of Medicinal Chemistry , 155 :171 , 2018 |
| Abstract : |
| PubMedSearch : Tintas_2018_Eur.J.Med.Chem_155_171 |
| PubMedID: 29886321 |