CHEMBL5206601
compound 5l showed potent cholinesterase inhibitory activity (IC50, 12.1 nM for eeAChE, 8.6 nM for hAChE, 2.6 muM for eqBuChE and 4.4 muM for hBuChE)
General
Type : anti-Abeta-aggregation, Multitarget, Pyridine
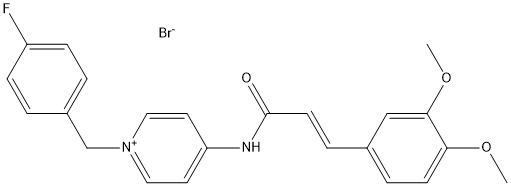
Chemical_Nomenclature : (E)-3-(3,4-dimethoxyphenyl)-N-[1-[(4-fluorophenyl)methyl]pyridin-1-ium-4-yl]prop-2-enamide\;bromide
Canonical SMILES : COC1=C(C=C(C=C1)C=CC(=O)NC2=CC=[N+](C=C2)CC3=CC=C(C=C3)F)OC.[Br-]
InChI : InChI=1S\/C23H21FN2O3.BrH\/c1-28-21-9-5-17(15-22(21)29-2)6-10-23(27)25-20-11-13-26(14-12-20)16-18-3-7-19(24)8-4-18\;\/h3-15H,16H2,1-2H3\;1H\/b10-6+\;
InChIKey : GTNZQLOSWVNPEP-AAGWESIMSA-N
Other name(s) : CHEMBL5206601 || BDBM50587756 || (e)-4-(3-(3,4-dimethoxyphenyl)acrylamido)-1-(4-fluorobenzyl) pyridin-1-ium bromide || (E)-3-(3,4-dimethoxyphenyl)-N-[1-[(4-fluorophenyl)methyl]pyridin-1-ium-4-yl]prop-2-enamide
MW : 473.3
Formula : C23H22BrFN2O3
CAS_number :
PubChem : 155293041, 155293042
UniChem : GTNZQLOSWVNPEP-AAGWESIMSA-N
Target
Families : CHEMBL5206601 ligand of proteins in family
ACHE
Protein :
human-ACHE
References (2)
| Title : QSAR analysis of the acetylcholinesterase inhibitory activity of some tertiary amine derivatives of cinnamic acid - Nekoeinia_2021_Struct.Chem_32_1123 |
| Author(s) : Nekoeinia M , Yousefinejad S |
| Ref : Struct Chem , 32 :1123 , 2021 |
| Abstract : |
| PubMedSearch : Nekoeinia_2021_Struct.Chem_32_1123 |
| PubMedID: |
| Title : Design, synthesis and evaluation of novel cinnamic acid derivatives bearing N-benzyl pyridinium moiety as multifunctional cholinesterase inhibitors for Alzheimer's disease - Lan_2017_J.Enzyme.Inhib.Med.Chem_32_776 |
| Author(s) : Lan JS , Hou JW , Liu Y , Ding Y , Zhang Y , Li L , Zhang T |
| Ref : J Enzyme Inhib Med Chem , 32 :776 , 2017 |
| Abstract : |
| PubMedSearch : Lan_2017_J.Enzyme.Inhib.Med.Chem_32_776 |
| PubMedID: 28585866 |