CHMP-thiocholine
can be synthetized as (Sp) or (P-) and (Rp) or (P+) stereoisomers
General
Type : Organophosphate, Trimethylammonium, Ammonium, Sulfur Compound
Chemical_Nomenclature : 2-(cycloheptyloxy-methyl-oxidophosphaniumyl)sulfanylethyl-trimethylazanium
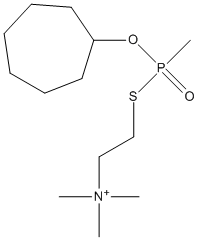
Canonical SMILES : C1CCC(CCC1)O[P](C)(=O)SCC[N+](C)(C)C
InChI : InChI=1S\/C13H29NO2PS\/c1-14(2,3)11-12-18-17(4,15)16-13-9-7-5-6-8-10-13\/h13H,5-12H2,1-4H3\/q+1
InChIKey : GLKKOOIXGTWDCJ-UHFFFAOYSA-N
Other name(s) : Cycloheptyl methylphosphonyl thiocholine
MW : 294.41
Formula : C13H29NO2PS
CAS_number :
PubChem : 101255751
UniChem : GLKKOOIXGTWDCJ-UHFFFAOYSA-N
Target
Families : CHMP-thiocholine ligand of proteins in family
ACHE
References (9)
| Title : Aspartate 74 as a primary determinant in acetylcholinesterase governing specificity to cationic organophosphonates - Hosea_1996_Biochemistry_35_10995 |
| Author(s) : Hosea NA , Radic Z , Tsigelny I , Berman HA , Quinn DM , Taylor P |
| Ref : Biochemistry , 35 :10995 , 1996 |
| Abstract : |
| PubMedSearch : Hosea_1996_Biochemistry_35_10995 |
| PubMedID: 8718893 |
| Title : Aspartate 74 as a primary determinant in acetylcholinesterase governing specificity to cationic organophosphonates - Hosea_1996_Biochemistry_35_10995 |
| Author(s) : Hosea NA , Radic Z , Tsigelny I , Berman HA , Quinn DM , Taylor P |
| Ref : Biochemistry , 35 :10995 , 1996 |
| Abstract : |
| PubMedSearch : Hosea_1996_Biochemistry_35_10995 |
| PubMedID: 8718893 |
| Title : Aspartate 74 as a primary determinant in acetylcholinesterase governing specificity to cationic organophosphonates - Hosea_1996_Biochemistry_35_10995 |
| Author(s) : Hosea NA , Radic Z , Tsigelny I , Berman HA , Quinn DM , Taylor P |
| Ref : Biochemistry , 35 :10995 , 1996 |
| Abstract : |
| PubMedSearch : Hosea_1996_Biochemistry_35_10995 |
| PubMedID: 8718893 |
| Title : Chiral reactions of acetylcholinesterase probed with enantiomeric methylphosphonothioates. Noncovalent determinants of enzyme chirality [published erratum appears in J Biol Chem 1989 Nov 25\;264(33):20154] - Berman_1989_J.Biol.Chem_264_3942 |
| Author(s) : Berman HA , Leonard K |
| Ref : Journal of Biological Chemistry , 264 :3942 , 1989 |
| Abstract : |
| PubMedSearch : Berman_1989_J.Biol.Chem_264_3942 |
| PubMedID: 2917983 |
| Title : Chiral nature of covalent methylphosphonyl conjugates of acetylcholinesterase - Berman_1989_J.Biol.Chem_264_3951 |
| Author(s) : Berman HA , Decker MM |
| Ref : Journal of Biological Chemistry , 264 :3951 , 1989 |
| Abstract : |
| PubMedSearch : Berman_1989_J.Biol.Chem_264_3951 |
| PubMedID: 2917984 |
| Title : Chiral reactions of acetylcholinesterase probed with enantiomeric methylphosphonothioates. Noncovalent determinants of enzyme chirality [published erratum appears in J Biol Chem 1989 Nov 25\;264(33):20154] - Berman_1989_J.Biol.Chem_264_3942 |
| Author(s) : Berman HA , Leonard K |
| Ref : Journal of Biological Chemistry , 264 :3942 , 1989 |
| Abstract : |
| PubMedSearch : Berman_1989_J.Biol.Chem_264_3942 |
| PubMedID: 2917983 |
| Title : Chiral nature of covalent methylphosphonyl conjugates of acetylcholinesterase - Berman_1989_J.Biol.Chem_264_3951 |
| Author(s) : Berman HA , Decker MM |
| Ref : Journal of Biological Chemistry , 264 :3951 , 1989 |
| Abstract : |
| PubMedSearch : Berman_1989_J.Biol.Chem_264_3951 |
| PubMedID: 2917984 |
| Title : Chiral reactions of acetylcholinesterase probed with enantiomeric methylphosphonothioates. Noncovalent determinants of enzyme chirality [published erratum appears in J Biol Chem 1989 Nov 25\;264(33):20154] - Berman_1989_J.Biol.Chem_264_3942 |
| Author(s) : Berman HA , Leonard K |
| Ref : Journal of Biological Chemistry , 264 :3942 , 1989 |
| Abstract : |
| PubMedSearch : Berman_1989_J.Biol.Chem_264_3942 |
| PubMedID: 2917983 |
| Title : Chiral nature of covalent methylphosphonyl conjugates of acetylcholinesterase - Berman_1989_J.Biol.Chem_264_3951 |
| Author(s) : Berman HA , Decker MM |
| Ref : Journal of Biological Chemistry , 264 :3951 , 1989 |
| Abstract : |
| PubMedSearch : Berman_1989_J.Biol.Chem_264_3951 |
| PubMedID: 2917984 |