Oprea1_491201
Ki hCA I 79.75 +/- 12.67 nM; hCA II 35.01 +/- 8.33 nM; AChE 19.58 +/- 3.28 nM
General
Type : Thiazole, Carbonic anhydrase inhibitor, Multitarget, Sulfur Compound, Sulfonamide
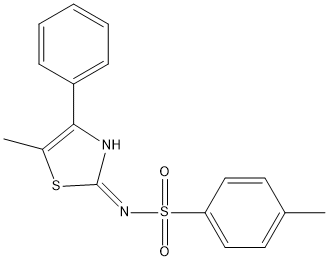
Chemical_Nomenclature : 4-methyl-N-(5-methyl-4-phenyl-1,3-thiazol-2-yl)benzenesulfonamide
Canonical SMILES : CC1=CC=C(C=C1)S(=O)(=O)NC2=NC(=C(S2)C)C3=CC=CC=C3
InChI : InChI=1S\/C17H16N2O2S2\/c1-12-8-10-15(11-9-12)23(20,21)19-17-18-16(13(2)22-17)14-6-4-3-5-7-14\/h3-11H,1-2H3,(H,18,19)
InChIKey : IUJWMEBGHYORJN-UHFFFAOYSA-N
Other name(s) : Compound 12 || AKOS003866800 || 4-methyl-N-(5-methyl-4-phenylthiazol-2-yl)benzenesulfonamide
MW : 344.5
Formula : C17H16N2O2S2
CAS_number :
PubChem : 3974857
UniChem : IUJWMEBGHYORJN-UHFFFAOYSA-N
Target
Families : Oprea1_491201 ligand of proteins in family
ACHE
Protein :
human-ACHE
References (3)
| Title : Synthesis of N-substituted 4-phenyl-2-aminothiazole derivatives and investigation of their inhibition properties against hCA I, II, and AChE enzymes - Bicer_2024_Arch.Biochem.Biophys__110159 |
| Author(s) : Bicer A , Caglayan C , Demir Y , Turkes C , Altundas R , Akyildiz H , Beydemir S |
| Ref : Archives of Biochemistry & Biophysics , :110159 , 2024 |
| Abstract : |
| PubMedSearch : Bicer_2024_Arch.Biochem.Biophys__110159 |
| PubMedID: 39322099 |
| Title : Synthesis of N-substituted 4-phenyl-2-aminothiazole derivatives and investigation of their inhibition properties against hCA I, II, and AChE enzymes - Bicer_2024_Arch.Biochem.Biophys__110159 |
| Author(s) : Bicer A , Caglayan C , Demir Y , Turkes C , Altundas R , Akyildiz H , Beydemir S |
| Ref : Archives of Biochemistry & Biophysics , :110159 , 2024 |
| Abstract : |
| PubMedSearch : Bicer_2024_Arch.Biochem.Biophys__110159 |
| PubMedID: 39322099 |
| Title : Synthesis of N-substituted 4-phenyl-2-aminothiazole derivatives and investigation of their inhibition properties against hCA I, II, and AChE enzymes - Bicer_2024_Arch.Biochem.Biophys__110159 |
| Author(s) : Bicer A , Caglayan C , Demir Y , Turkes C , Altundas R , Akyildiz H , Beydemir S |
| Ref : Archives of Biochemistry & Biophysics , :110159 , 2024 |
| Abstract : |
| PubMedSearch : Bicer_2024_Arch.Biochem.Biophys__110159 |
| PubMedID: 39322099 |