STL118972
IC50 hBCHE 0.0465 microM no inhibition of AChE at 50 microM
General
Type : Sulfonamide, Sulfur Compound, Benzenesulfonamide
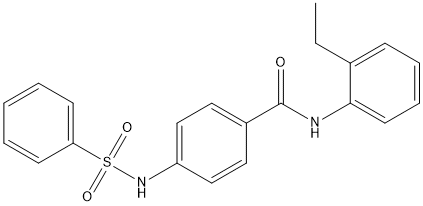
Chemical_Nomenclature : 4-(benzenesulfonamido)-N-(2-ethylphenyl)benzamide
Canonical SMILES : CCC1=CC=CC=C1NC(=O)C2=CC=C(C=C2)NS(=O)(=O)C3=CC=CC=C3
InChI : InChI=1S\/C21H20N2O3S\/c1-2-16-8-6-7-11-20(16)22-21(24)17-12-14-18(15-13-17)23-27(25,26)19-9-4-3-5-10-19\/h3-15,23H,2H2,1H3,(H,22,24)
InChIKey : YCLYWWBNCQGCMD-UHFFFAOYSA-N
Other name(s) : AKOS000394542 || N-(2-ethylphenyl)-4-[(phenylsulfonyl)amino]benzamide || Z57637739
MW : 380.5
Formula : C21H20N2O3S
CAS_number :
PubChem : 1261132
UniChem : YCLYWWBNCQGCMD-UHFFFAOYSA-N
Target
Families : STL118972 ligand of proteins in family
BCHE
Protein :
human-BCHE
References (3)
| Title : Identification of sulfonamide-based butyrylcholinesterase inhibitors using machine learning - Ganeshpurkar_2022_Future.Med.Chem__ |
| Author(s) : Ganeshpurkar A , Singh R , Kumar D , Gutti G , Gore P , Sahu B , Kumar A , Singh SK |
| Ref : Future Med Chem , : , 2022 |
| Abstract : |
| PubMedSearch : Ganeshpurkar_2022_Future.Med.Chem__ |
| PubMedID: 35707942 |
| Title : Identification of sulfonamide-based butyrylcholinesterase inhibitors using machine learning - Ganeshpurkar_2022_Future.Med.Chem__ |
| Author(s) : Ganeshpurkar A , Singh R , Kumar D , Gutti G , Gore P , Sahu B , Kumar A , Singh SK |
| Ref : Future Med Chem , : , 2022 |
| Abstract : |
| PubMedSearch : Ganeshpurkar_2022_Future.Med.Chem__ |
| PubMedID: 35707942 |
| Title : Identification of sulfonamide-based butyrylcholinesterase inhibitors using machine learning - Ganeshpurkar_2022_Future.Med.Chem__ |
| Author(s) : Ganeshpurkar A , Singh R , Kumar D , Gutti G , Gore P , Sahu B , Kumar A , Singh SK |
| Ref : Future Med Chem , : , 2022 |
| Abstract : |
| PubMedSearch : Ganeshpurkar_2022_Future.Med.Chem__ |
| PubMedID: 35707942 |