Tetrahydrofurobenzofuran-cymserine
General
Type : Derivative of physostigmine-eserine, Carbamate, Benzofuran
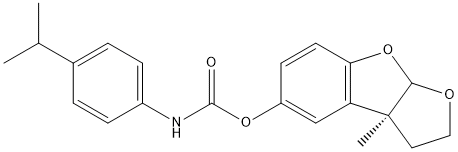
Chemical_Nomenclature : [(8bS)-8b-methyl-2,3a-dihydro-1H-furo[2,3-b][1]benzofuran-7-yl] N-(4-propan-2-ylphenyl)carbamate
Canonical SMILES : CC(C)C1=CC=C(C=C1)NC(=O)OC2=CC3=C(C=C2)OC4C3(CCO4)C
InChI : InChI=1S\/C21H23NO4\/c1-13(2)14-4-6-15(7-5-14)22-20(23)25-16-8-9-18-17(12-16)21(3)10-11-24-19(21)26-18\/h4-9,12-13,19H,10-11H2,1-3H3,(H,22,23)\/t19?,21-\/m0\/s1
InChIKey : QIYIAVWWIGXXBQ-QWAKEFERSA-N
Other name(s) : Tetrahydrofurobenzofuran cymserine || CHEMBL1242981 || BDBM10626 || Tetrahydrofurobenzofuran Carbamate 10a || (-)-(3aS)-3a-methyl-2,3,3a,8a-Tetrahydrofuro[2,3-b]benzofuran-5-yl N-(p-Isopropylphenyl)carbamate || (2S)-2-methyl-5,7-dioxatricyclo[6.4.0.0^{2,6}]dodeca-1(12),8,10-trien-11-yl N-[4-(propan-2-yl)phenyl]carbamate || (-) cymyl carbamate of tetrahydrofurobenzofurol
MW : 353.4
Formula : C21H23NO4
CAS_number :
PubChem : 23645292
UniChem : QIYIAVWWIGXXBQ-QWAKEFERSA-N
Target
Families : Tetrahydrofurobenzofuran-cymserine ligand of proteins in family
ACHE
Protein :
human-ACHE
References (2)
| Title : Accommodation of physostigmine and its analogues by acetylcholinesterase is dominated by hydrophobic interactions - Barak_2009_Biochem.J_417_213 |
| Author(s) : Barak D , Ordentlich A , Stein D , Yu QS , Greig NH , Shafferman A |
| Ref : Biochemical Journal , 417 :213 , 2009 |
| Abstract : |
| PubMedSearch : Barak_2009_Biochem.J_417_213 |
| PubMedID: 18729824 |
| Title : Tetrahydrofurobenzofuran cymserine, a potent butyrylcholinesterase inhibitor and experimental Alzheimer drug candidate, enzyme kinetic analysis - Kamal_2008_J.Neural.Transm.(Vienna)_115_889 |
| Author(s) : Kamal MA , Qu X , Yu QS , Tweedie D , Holloway HW , Li Y , Tan Y , Greig NH |
| Ref : J Neural Transm (Vienna) , 115 :889 , 2008 |
| Abstract : |
| PubMedSearch : Kamal_2008_J.Neural.Transm.(Vienna)_115_889 |
| PubMedID: 18235987 |