Procainamide
Analog of procaine. A derivative of Procaine with less CNS action.Used in affinity chromatography to purify cholinesterases
General
Type : Local anesthetic,Not A\/B H target,Anti-Arrhythmia Agents,Benzamide
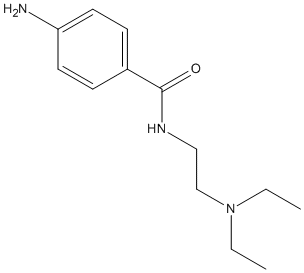
Chemical_Nomenclature : 4-Amino-N-[2-(diethylamino) ethyl]benzamide monohydrochloride
Canonical SMILES : CCN(CC)CCNC(=O)C1=CC=C(C=C1)N
InChI : InChI=1S\/C13H21N3O\/c1-3-16(4-2)10-9-15-13(17)11-5-7-12(14)8-6-11\/h5-8H,3-4,9-10,14H2,1-2H3,(H,15,17)
InChIKey : REQCZEXYDRLIBE-UHFFFAOYSA-N
Other name(s) : Novocainamide,Novocamid,Procamide,Pronestyl,Biocoryl,Novocaine amide,Procaine amide,Procapan,Procan,Promine,Procaineamide,Procan SR
MW : 271.79
Formula : C13H21N3O.HCl
CAS_number : 51-06-9 || 614-39-1
PubChem : 4913
UniChem : REQCZEXYDRLIBE-UHFFFAOYSA-N
IUPHAR : 4811
Wikipedia : Procainamide
Target
References (14)
| Title : Acetylcholinesterase in Dendrobaena veneta (Oligochaeta: Opisthopora) is present with forms sensitive and insensitive to phosphatidylinositol phospholipase C. Biochemical characterization and histochemical localization in the nervous system - Talesa_1996_Eur.J.Biochem_238_538 |
| Author(s) : Talesa V , Romani R , Rosi G , Giovannini E |
| Ref : European Journal of Biochemistry , 238 :538 , 1996 |
| Abstract : Talesa_1996_Eur.J.Biochem_238_538 |
| ESTHER : Talesa_1996_Eur.J.Biochem_238_538 |
| PubMedSearch : Talesa_1996_Eur.J.Biochem_238_538 |
| PubMedID: 8681969 |
| Title : Comparison of standard chromatographic procedures for the optimal purification of soluble human brain acetylcholinesterase - Novales-Li_1994_Biomedic.Chromat_8_259 |
| Author(s) : Novales-Li P |
| Ref : Biomedical Chromatography , 8 :259 , 1994 |
| Abstract : Novales-Li_1994_Biomedic.Chromat_8_259 |
| ESTHER : Novales-Li_1994_Biomedic.Chromat_8_259 |
| PubMedSearch : Novales-Li_1994_Biomedic.Chromat_8_259 |
| PubMedID: 7888726 |
| Title : Sheep brain pseudocholinesterase: inhibition kinetics of the partially purified enzyme by some substrate analogues - Cokugras_1993_Chem.Biol.Interact_87_259 |
| Author(s) : Cokugras AN , Tezcan EF |
| Ref : Chemico-Biological Interactions , 87 :259 , 1993 |
| Abstract : Cokugras_1993_Chem.Biol.Interact_87_259 |
| ESTHER : Cokugras_1993_Chem.Biol.Interact_87_259 |
| PubMedSearch : Cokugras_1993_Chem.Biol.Interact_87_259 |
| PubMedID: 8343984 |
| Title : Isolation of a tripeptide (Ala-Gly-Ser) exhibiting weak acetylthiocholine hydrolyzing activity from a high-salt soluble form of monkey diaphragm acetylcholinesterase - Jayanthi_1992_Neurochem.Res_17_351 |
| Author(s) : Jayanthi LD , Balasubramanian AS |
| Ref : Neurochemical Research , 17 :351 , 1992 |
| Abstract : Jayanthi_1992_Neurochem.Res_17_351 |
| ESTHER : Jayanthi_1992_Neurochem.Res_17_351 |
| PubMedSearch : Jayanthi_1992_Neurochem.Res_17_351 |
| PubMedID: 1513418 |
| Title : Cholinesterases exhibiting aryl acylamidase activity in human amniotic fluid - Jayanthi_1992_Clin.Chim.Acta_205_157 |
| Author(s) : Jayanthi LD , Balasubramanian N , Balasubramanian AS |
| Ref : Clinica Chimica Acta , 205 :157 , 1992 |
| Abstract : Jayanthi_1992_Clin.Chim.Acta_205_157 |
| ESTHER : Jayanthi_1992_Clin.Chim.Acta_205_157 |
| PubMedSearch : Jayanthi_1992_Clin.Chim.Acta_205_157 |
| PubMedID: 1349516 |
| Title : Ligand stabilization of cholinesterases - Payne_1989_Biochim.Biophys.Acta_999_46 |
| Author(s) : Payne CS , Saeed M , Wolfe AD |
| Ref : Biochimica & Biophysica Acta , 999 :46 , 1989 |
| Abstract : Payne_1989_Biochim.Biophys.Acta_999_46 |
| ESTHER : Payne_1989_Biochim.Biophys.Acta_999_46 |
| PubMedSearch : Payne_1989_Biochim.Biophys.Acta_999_46 |
| PubMedID: 2804138 |
| Title : The effect of procainamide on plasma cholinesterase activity - Kambam_1987_Can.J.Anaesth_34_579 |
| Author(s) : Kambam JR , Naukam RJ , Sastry BV |
| Ref : Canadian Journal of Anaesthesia , 34 :579 , 1987 |
| Abstract : Kambam_1987_Can.J.Anaesth_34_579 |
| ESTHER : Kambam_1987_Can.J.Anaesth_34_579 |
| PubMedSearch : Kambam_1987_Can.J.Anaesth_34_579 |
| PubMedID: 3677282 |
| Title : A peptidase activity exhibited by human serum pseudocholinesterase - Boopathy_1987_Eur.J.Biochem_162_191 |
| Author(s) : Boopathy R , Balasubramanian AS |
| Ref : European Journal of Biochemistry , 162 :191 , 1987 |
| Abstract : Boopathy_1987_Eur.J.Biochem_162_191 |
| ESTHER : Boopathy_1987_Eur.J.Biochem_162_191 |
| PubMedSearch : Boopathy_1987_Eur.J.Biochem_162_191 |
| PubMedID: 3545820 |
| Title : A simplified procedure for the purification of large quantities of fetal bovine serum acetylcholinesterase - De La Hoz_1986_Life.Sci_39_195 |
| Author(s) : De La Hoz DM , Doctor BP , Ralston JS , Rush RS , Wolfe AD |
| Ref : Life Sciences , 39 :195 , 1986 |
| Abstract : De La Hoz_1986_Life.Sci_39_195 |
| ESTHER : De La Hoz_1986_Life.Sci_39_195 |
| PubMedSearch : De La Hoz_1986_Life.Sci_39_195 |
| PubMedID: 3736320 |
| Title : Acetylcholinesterase from fetal bovine serum. Purification and characterization of soluble G4 enzyme - Ralston_1985_J.Biol.Chem_260_4312 |
| Author(s) : Ralston JS , Rush RS , Doctor BP , Wolfe AD |
| Ref : Journal of Biological Chemistry , 260 :4312 , 1985 |
| Abstract : Ralston_1985_J.Biol.Chem_260_4312 |
| ESTHER : Ralston_1985_J.Biol.Chem_260_4312 |
| PubMedSearch : Ralston_1985_J.Biol.Chem_260_4312 |
| PubMedID: 3980478 |
| Title : Butyrylcholinesterase: inhibition by arsenite, fluoride, and other ligands, cooperativity in binding - Page_1985_Mol.Pharmacol_27_437 |
| Author(s) : Page JD , Wilson IB , Silman I |
| Ref : Molecular Pharmacology , 27 :437 , 1985 |
| Abstract : Page_1985_Mol.Pharmacol_27_437 |
| ESTHER : Page_1985_Mol.Pharmacol_27_437 |
| PubMedSearch : Page_1985_Mol.Pharmacol_27_437 |
| PubMedID: 3982389 |
| Title : [Affinity electrophoresis in polyacrylamide gel. Influence of the concentration in the gel on the apparent affinity of cholinesterase for an anionic ligand site]. [French] - Masson_1985_J.Chromato_328_135 |
| Author(s) : Masson P , Marnot B |
| Ref : Journal of Chromatography , 328 :135 , 1985 |
| Abstract : Masson_1985_J.Chromato_328_135 |
| ESTHER : Masson_1985_J.Chromato_328_135 |
| PubMedSearch : Masson_1985_J.Chromato_328_135 |
| PubMedID: 4030968 |
| Title : [Electrophoretic study of aged butyrylcholinesterase after inhibition by soman]. [French] - Masson_1984_Biochimie_66_235 |
| Author(s) : Masson P , Marnot B , Lombard JY , Morelis P |
| Ref : Biochimie , 66 :235 , 1984 |
| Abstract : Masson_1984_Biochimie_66_235 |
| ESTHER : Masson_1984_Biochimie_66_235 |
| PubMedSearch : Masson_1984_Biochimie_66_235 |
| PubMedID: 6331528 |
| Title : The aryl acylamidases and their relationship to cholinesterases in human serum, erythrocyte and liver - George_1981_Eur.J.Biochem_121_177 |
| Author(s) : George ST , Balasubramanian AS |
| Ref : European Journal of Biochemistry , 121 :177 , 1981 |
| Abstract : George_1981_Eur.J.Biochem_121_177 |
| ESTHER : George_1981_Eur.J.Biochem_121_177 |
| PubMedSearch : George_1981_Eur.J.Biochem_121_177 |
| PubMedID: 7035166 |