Profenofos
General
Type : Organophosphate,Insecticide,Sulfur Compound
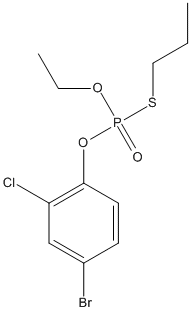
Chemical_Nomenclature : O-(4-Bromo-2-chlorophenyl)-O-ethyl-S-propyl phosphorothioate
Canonical SMILES : CCCSP(=O)(OCC)OC1=C(C=C(C=C1)Br)Cl
InChI : InChI=1S\/C11H15BrClO3PS\/c1-3-7-18-17(14,15-4-2)16-11-6-5-9(12)8-10(11)13\/h5-6,8H,3-4,7H2,1-2H3
InChIKey : QYMMJNLHFKGANY-UHFFFAOYSA-N
Other name(s) : Curacron,Bromo-2-chlorophenyl) O-ethyl S-propyl phosphorothioate,Polycron,Selecron,Profenophos
MW : 373.63
Formula : C11H15BrClO3PS
CAS_number : 41198-08-7
PubChem : 38779
UniChem : QYMMJNLHFKGANY-UHFFFAOYSA-N
IUPHAR :
Wikipedia : Profenofos
Target
Families : Profenofos ligand of proteins in family: ACHE || ACPH_Peptidase_S9
Stucture :
Protein : human-APEH
References (7)
| Title : Profenofos, an Acetylcholinesterase-Inhibiting Organophosphorus Pesticide: A Short Review of Its Usage, Toxicity, and Biodegradation - Kushwaha_2016_J.Environ.Qual_45_1478 |
| Author(s) : Kushwaha M , Verma S , Chatterjee S |
| Ref : J Environ Qual , 45 :1478 , 2016 |
| Abstract : Kushwaha_2016_J.Environ.Qual_45_1478 |
| ESTHER : Kushwaha_2016_J.Environ.Qual_45_1478 |
| PubMedSearch : Kushwaha_2016_J.Environ.Qual_45_1478 |
| PubMedID: 27695768 |
| Title : Blood acylpeptide hydrolase activity is a sensitive marker for exposure to some organophosphate toxicants - Quistad_2005_Toxicol.Sci_86_291 |
| Author(s) : Quistad GB , Klintenberg R , Casida JE |
| Ref : Toxicol Sci , 86 :291 , 2005 |
| Abstract : Quistad_2005_Toxicol.Sci_86_291 |
| ESTHER : Quistad_2005_Toxicol.Sci_86_291 |
| PubMedSearch : Quistad_2005_Toxicol.Sci_86_291 |
| PubMedID: 15888665 |
| Gene_locus related to this paper: human-APEH |
| Title : Organophosphorus pesticide-induced butyrylcholinesterase inhibition and potentiation of succinylcholine toxicity in mice - Sparks_1999_J.Biochem.Mol.Toxicol_13_113 |
| Author(s) : Sparks SE , Quistad GB , Casida JE |
| Ref : J Biochem Mol Toxicol , 13 :113 , 1999 |
| Abstract : Sparks_1999_J.Biochem.Mol.Toxicol_13_113 |
| ESTHER : Sparks_1999_J.Biochem.Mol.Toxicol_13_113 |
| PubMedSearch : Sparks_1999_J.Biochem.Mol.Toxicol_13_113 |
| PubMedID: 9890196 |
| Title : Hepatic injury and disturbed amino acid metabolism in mice following prolonged exposure to organophosphorus pesticides - Gomes_1999_Hum.Exp.Toxicol_18_33 |
| Author(s) : Gomes J , Dawodu AH , Lloyd O , Revitt DM , Anilal SV |
| Ref : Hum Exp Toxicol , 18 :33 , 1999 |
| Abstract : Gomes_1999_Hum.Exp.Toxicol_18_33 |
| ESTHER : Gomes_1999_Hum.Exp.Toxicol_18_33 |
| PubMedSearch : Gomes_1999_Hum.Exp.Toxicol_18_33 |
| PubMedID: 10025366 |
| Title : Profenofos insecticide bioactivation in relation to antidote action and the stereospecificity of acetylcholinesterase inhibition, reactivation, and aging - Glickman_1984_Toxicol.Appl.Pharmacol_73_16 |
| Author(s) : Glickman AH , Wing KD , Casida JE |
| Ref : Toxicology & Applied Pharmacology , 73 :16 , 1984 |
| Abstract : Glickman_1984_Toxicol.Appl.Pharmacol_73_16 |
| ESTHER : Glickman_1984_Toxicol.Appl.Pharmacol_73_16 |
| PubMedSearch : Glickman_1984_Toxicol.Appl.Pharmacol_73_16 |
| PubMedID: 6710513 |
| Title : Dislodgable insecticide residues on cotton foliage: fenvalarate, permethrin, sulprofos, chlorpyrifos, methyl parathion, EPN, oxamyl, and profenofos - |
| Author(s) : Buck NA , Estesen BJ , Ware GW |
| Ref : Bulletin of Environmental Contamination & Toxicology , 24 :283 , 1980 |
| PubMedID: 6153915 |
| Title : Dislodgable insecticide residues on cotton foliage: permethrin, curacron, fenvalarate, sulprofos, decis, and endosulfan - |
| Author(s) : Estesen BJ , Buck NA , Ware GW |
| Ref : Bulletin of Environmental Contamination & Toxicology , 22 :245 , 1979 |
| PubMedID: 465782 |