Pyranone-carbamate-cpd7p
IC50 hAChE 3.6 microM; IC50 hBChE 5.2 nM || hBChE IC50 = 9.12 nM (IC50eeAChE >20 microM) and anti-neuroinflammatory activity (NO inhibition = 28.82% at 10M, comparable to hydrocortisone
General
Type : Carbamate,Piperidine,Antioxidant
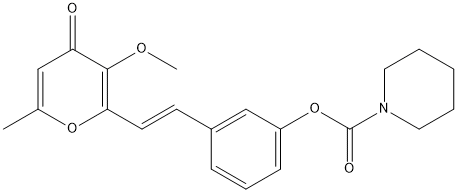
Chemical_Nomenclature : (E)-3-(2-(3-methoxy-6-methyl-4-oxo-4H-pyran-2-yl)vinyl)phenyl piperidine-1-carboxylate
Canonical SMILES : C1=C(OC(=C(C1=O)OC)C=CC2=CC(=CC=C2)OC(=O)N3CCCCC3)C
InChI : InChI=1S\/C21H23NO5\/c1-15-13-18(23)20(25-2)19(26-15)10-9-16-7-6-8-17(14-16)27-21(24)22-11-4-3-5-12-22\/h6-10,13-14H,3-5,11-12H2,1-2H3\/b10-9+
InChIKey : MOPDEKASFLNBJW-MDZDMXLPSA-N
Other name(s) :
MW : 369.41
Formula : C21H23NO5
CAS_number :
PubChem :
UniChem : MOPDEKASFLNBJW-MDZDMXLPSA-N
IUPHAR :
Wikipedia :
Target
Families : Pyranone-carbamate-cpd7p ligand of proteins in family: BCHE
Stucture :
Protein : human-BCHE
References (1)
| Title : Novel anti-neuroinflammatory pyranone-carbamate derivatives as selective butyrylcholinesterase inhibitors for treating Alzheimer's disease - Yu_2024_J.Enzyme.Inhib.Med.Chem_39_2313682 |
| Author(s) : Yu C , Liu X , Ma B , Xu J , Chen Y , Dai C , Peng H , Zha D |
| Ref : J Enzyme Inhib Med Chem , 39 :2313682 , 2024 |
| Abstract : Yu_2024_J.Enzyme.Inhib.Med.Chem_39_2313682 |
| ESTHER : Yu_2024_J.Enzyme.Inhib.Med.Chem_39_2313682 |
| PubMedSearch : Yu_2024_J.Enzyme.Inhib.Med.Chem_39_2313682 |
| PubMedID: 38362862 |