Sodium-dodecyl-sulfate
detergent and a protein denaturant. Sodium Lauryl Sulfate (SLS) is an anionic surfactant naturally derived from coconut or palm kernel oil
General
Type : Detergent,Sulfur Compound
Chemical_Nomenclature : sodium\;dodecyl sulfate
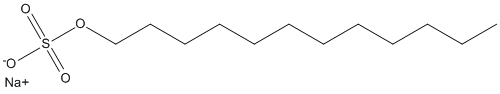
Canonical SMILES : CCCCCCCCCCCCOS(=O)(=O)[O-].[Na+]
InChI : InChI=1S\/C12H26O4S.Na\/c1-2-3-4-5-6-7-8-9-10-11-12-16-17(13,14)15\;\/h2-12H2,1H3,(H,13,14,15)\;\/q\;+1\/p-1
InChIKey : DBMJMQXJHONAFJ-UHFFFAOYSA-M
Other name(s) : Sodium lauryl sulphate,Sodium dodecyl sulphate,Sodium dodecylsulphate,SDS
MW : 288.38
Formula : C12H25O4S.Na or C12H25NaO4S
CAS_number : 151-21-3
PubChem : 3423265
UniChem : DBMJMQXJHONAFJ-UHFFFAOYSA-M
IUPHAR :
Wikipedia :
References (2)
| Title : Molecular Modeling Investigation of the Interaction between Humicola insolens Cutinase and SDS Surfactant Suggests a Mechanism for Enzyme Inactivation - Kjolbye_2019_J.Chem.Inf.Model_59_1977 |
| Author(s) : Kjolbye LR , Laustsen A , Vestergaard M , Periole X , De Maria L , Svendsen A , Coletta A , Schiott B |
| Ref : J Chem Inf Model , 59 :1977 , 2019 |
| Abstract : Kjolbye_2019_J.Chem.Inf.Model_59_1977 |
| ESTHER : Kjolbye_2019_J.Chem.Inf.Model_59_1977 |
| PubMedSearch : Kjolbye_2019_J.Chem.Inf.Model_59_1977 |
| PubMedID: 30844270 |
| Gene_locus related to this paper: humin-cut |
| Title : Thermodynamic and structural investigation of the specific SDS binding of humicola insolens cutinase - Kold_2014_Protein.Sci_23_1023 |
| Author(s) : Kold D , Dauter Z , Laustsen AK , Brzozowski AM , Turkenburg JP , Nielsen AD , Koldso H , Petersen E , Schiott B , De Maria L , Wilson KS , Svendsen A , Wimmer R |
| Ref : Protein Science , 23 :1023 , 2014 |
| Abstract : Kold_2014_Protein.Sci_23_1023 |
| ESTHER : Kold_2014_Protein.Sci_23_1023 |
| PubMedSearch : Kold_2014_Protein.Sci_23_1023 |
| PubMedID: 24832484 |
| Gene_locus related to this paper: humin-cut |