t-AUCMB
Comparable cytotoxicity to sorafenib, but without inhibitory activity against the primary targets of sorafenib, such as C-Raf kinase and vascular endothelial growth factor receptor 2 (VEGFR2) kinase
General
Type : Urea derivative,Multitarget,Adamantyl
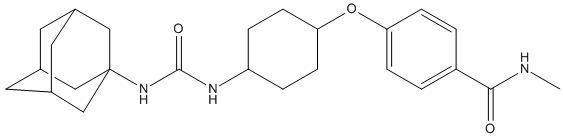
Chemical_Nomenclature : 4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-N-methyl-benzamide
Canonical SMILES : CNC(=O)C1=CC=C(C=C1)OC2CCC(CC2)NC(=O)NC34CC5CC(C3)CC(C5)C4
InChI : InChI=1S\/C25H35N3O3\/c1-26-23(29)19-2-6-21(7-3-19)31-22-8-4-20(5-9-22)27-24(30)28-25-13-16-10-17(14-25)12-18(11-16)15-25\/h2-3,6-7,16-18,20,22H,4-5,8-15H2,1H3,(H,26,29)(H2,27,28,30)
InChIKey : UHCRWCSTKBZBFL-UHFFFAOYSA-N
Other name(s) : CHEMBL2397139,SCHEMBL15272724,SCHEMBL15272793,SCHEMBL17431705,BDBM410785,4-[4-(1-adamantylcarbamoylamino)cyclohexyl]oxy-N-methylbenzamide
MW : 425.6
Formula : C25H35N3O3
CAS_number :
PubChem : 71682499
UniChem : UHCRWCSTKBZBFL-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : t-AUCMB ligand of proteins in family: Epoxide_hydrolase
Stucture :
Protein : human-EPHX2 || mouse-hyes
References (1)
| Title : Novel sorafenib-based structural analogues: in-vitro anticancer evaluation of t-MTUCB and t-AUCMB - Wecksler_2014_Anticancer.Drugs_25_433 |
| Author(s) : Wecksler AT , Hwang SH , Wettersten HI , Gilda JE , Patton A , Leon LJ , Carraway KL, 3rd , Gomes AV , Baar K , Weiss RH , Hammock BD |
| Ref : Anticancer Drugs , 25 :433 , 2014 |
| Abstract : Wecksler_2014_Anticancer.Drugs_25_433 |
| ESTHER : Wecksler_2014_Anticancer.Drugs_25_433 |
| PubMedSearch : Wecksler_2014_Anticancer.Drugs_25_433 |
| PubMedID: 24525589 |