Dihydroprecondylocarpine-acetate
Dihydroprecondylocarpine acetate is an organic cation which is an intermediate in the biosynthetic pathway leading to the synthesis of the monoterpenoid indole alkaloids, catharanthine and tabersonine. Leads to the formation of Dehydrosecodine an instable intermediate in the biosynthesis of aspidosperma and iboga alkaloids. It is a terpenoid indole alkaloid, a methyl ester, a dihydropyridine, a member of indoles, an alkaloid ester and an enamine. Three carboxylesterase (CXE)-like enzymes from Catharanthus roseus and Tabernanthe iboga catalyze regio- and enantiodivergent cycloaddition reactions to generate the aspidosperma (tabersonine synthase, TS) and iboga (coronaridine synthase, CorS; catharanthine synthase, CS) scaffolds from a common biosynthetic intermediate. Dehydrosecodine(1+) (CID 135398075) is a tertiary ammonium ion result from the protonation of the tertiary amino group of dehydrosecodine (the enamine form). Dehydrosecodine is the substrate and tabersonine catharanthine and carbomehtoxycleaviminium are the products of these enzyme. These later compound are substrate for aditional enzymes to give vinblastine coronaridine and ibogaine
General
Type : Indole, Alkaloid, Natural
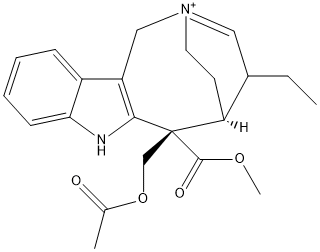
Chemical_Nomenclature : methyl (1S,2S)-2-(acetyloxymethyl)-16-ethyl-4-aza-14-azoniatetracyclo[12.2.2.03,11.05,10]octadeca-3(11),5,7,9,14-pentaene-2-carboxylate
Canonical SMILES : CCC1C=[N+]2CCC1C(C3=C(CC2)C4=CC=CC=C4N3)(COC(=O)C)C(=O)OC
InChI : InChI=1S\/C23H29N2O4\/c1-4-16-13-25-11-9-18-17-7-5-6-8-20(17)24-21(18)23(22(27)28-3,14-29-15(2)26)19(16)10-12-25\/h5-8,13,16,19,24H,4,9-12,14H2,1-3H3\/q+1\/t16?,19-,23-\/m0\/s1
InChIKey : QXQMGTOURBIIKD-RUMUXARQSA-N
Other name(s) : dihydroprecondylocarpine acetate || CHEBI:142770 || (6S,7S)-7-(acetoxymethyl)-5-ethyl-7-(methoxycarbonyl)-1,2,5,6,7,8-hexahydro-3,6-ethanoazonino[5,4-b]indol-3-ium
MW : 397.5
Formula : C23H29N2O4+
CAS_number :
PubChem : 135398095
UniChem : QXQMGTOURBIIKD-RUMUXARQSA-N
Target
Structures : No structure
Families : Plant_carboxylesterase
References (4)
| Title : Evolution and diversification of carboxylesterase-like [4+2] cyclases in aspidosperma and iboga alkaloid biosynthesis - DeMars_2024_Proc.Natl.Acad.Sci.U.S.A_121_e2318586121 |
| Author(s) : DeMars MD, 2nd , O'Connor SE |
| Ref : Proc Natl Acad Sci U S A , 121 :e2318586121 , 2024 |
| Abstract : |
| PubMedSearch : DeMars_2024_Proc.Natl.Acad.Sci.U.S.A_121_e2318586121 |
| PubMedID: 38319969 |
| Gene_locus related to this paper: catro-CS , catro-TS , tabib-a0a5b8x6s2 , tabib-CorS |
| Title : Structural basis of cycloaddition in biosynthesis of iboga and aspidosperma alkaloids - Caputi_2020_Nat.Chem.Biol_16_383 |
| Author(s) : Caputi L , Franke J , Bussey K , Farrow SC , Vieira IJC , Stevenson CEM , Lawson DM , O'Connor SE |
| Ref : Nat Chemical Biology , 16 :383 , 2020 |
| Abstract : |
| PubMedSearch : Caputi_2020_Nat.Chem.Biol_16_383 |
| PubMedID: 32066966 |
| Gene_locus related to this paper: catro-CS , catro-TS , tabib-a0a5b8x6s2 , tabib-CorS |
| Title : Completion of the canonical pathway for assembly of anticancer drugs vincristine\/vinblastine in Catharanthus roseus - Qu_2019_Plant.J_97_257 |
| Author(s) : Qu Y , Safonova O , De Luca V |
| Ref : Plant J , 97 :257 , 2019 |
| Abstract : |
| PubMedSearch : Qu_2019_Plant.J_97_257 |
| PubMedID: 30256480 |
| Gene_locus related to this paper: catro-hl3 , catro-hl4 |
| Title : Solution of the multistep pathway for assembly of corynanthean, strychnos, iboga, and aspidosperma monoterpenoid indole alkaloids from 19E-geissoschizine - Qu_2018_Proc.Natl.Acad.Sci.U.S.A_115_3180 |
| Author(s) : Qu Y , Easson M , Simionescu R , Hajicek J , Thamm AMK , Salim V , De Luca V |
| Ref : Proc Natl Acad Sci U S A , 115 :3180 , 2018 |
| Abstract : |
| PubMedSearch : Qu_2018_Proc.Natl.Acad.Sci.U.S.A_115_3180 |
| PubMedID: 29511102 |
| Gene_locus related to this paper: catro-CS , catro-TS , catro-hl3 , catro-hl4 |