N-benzyl-picolinamide
General
Type : Pyridine, Carboxamide
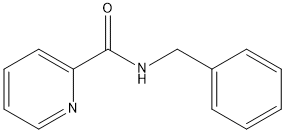
Chemical_Nomenclature : N-benzylpyridine-2-carboxamide
Canonical SMILES : C1=CC=C(C=C1)CNC(=O)C2=CC=CC=N2
InChI : InChI=1S\/C13H12N2O\/c16-13(12-8-4-5-9-14-12)15-10-11-6-2-1-3-7-11\/h1-9H,10H2,(H,15,16)
InChIKey : NNIFYECULHSHBH-UHFFFAOYSA-N
Other name(s) : N-benzylpyridine-2-carboxamide || N-Benzylpicolinamide || 2-Pyridinecarboxamide, N-(phenylmethyl)- || starbld0037443 || Cambridge id 5100084 || 2-(N-benzylcarbamoyl)pyridine || n-benzyl-2-pyridinecarboxamide || SCHEMBL3083971
MW : 212.25
Formula : C13H12N2O
CAS_number : 18904-38-6
PubChem : 687032
UniChem : NNIFYECULHSHBH-UHFFFAOYSA-N
Target
Structures : 8P8F
Families : Hormone-sensitive_lipase_like
References (3)
| Title : Triphenylphosphine-catalysed amide bond formation between carboxylic acids and amines - Lenstra_2014_Chem.Commun.(Camb)_50_5763 |
| Author(s) : Lenstra DC , Rutjes FP , Mecinovic J |
| Ref : Chem Commun (Camb) , 50 :5763 , 2014 |
| Abstract : |
| PubMedSearch : Lenstra_2014_Chem.Commun.(Camb)_50_5763 |
| PubMedID: 24752820 |
| Title : Triphenylphosphine-catalysed amide bond formation between carboxylic acids and amines - Lenstra_2014_Chem.Commun.(Camb)_50_5763 |
| Author(s) : Lenstra DC , Rutjes FP , Mecinovic J |
| Ref : Chem Commun (Camb) , 50 :5763 , 2014 |
| Abstract : |
| PubMedSearch : Lenstra_2014_Chem.Commun.(Camb)_50_5763 |
| PubMedID: 24752820 |
| Title : Triphenylphosphine-catalysed amide bond formation between carboxylic acids and amines - Lenstra_2014_Chem.Commun.(Camb)_50_5763 |
| Author(s) : Lenstra DC , Rutjes FP , Mecinovic J |
| Ref : Chem Commun (Camb) , 50 :5763 , 2014 |
| Abstract : |
| PubMedSearch : Lenstra_2014_Chem.Commun.(Camb)_50_5763 |
| PubMedID: 24752820 |