Pentagalloylglucose
General
Type : Derivative of Gallate || Natural || Polyphenol
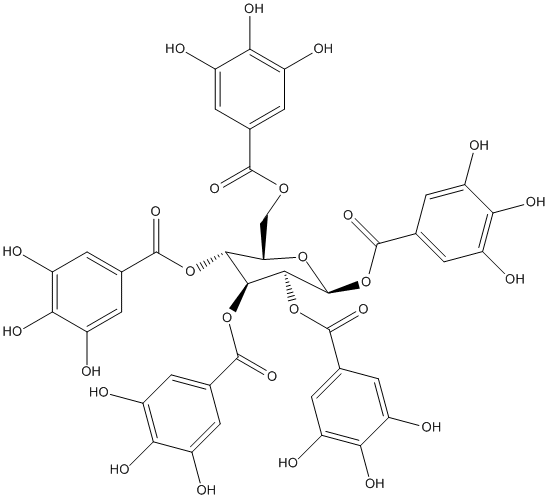
Chemical_Nomenclature : [(2R,3R,4S,5R,6S)-3,4,5,6-tetrakis[(3,4,5-trihydroxybenzoyl)oxy]oxan-2-yl]methyl 3,4,5-trihydroxybenzoate
Canonical SMILES : C1=C(C=C(C(=C1O)O)O)C(=O)OCC2C(C(C(C(O2)OC(=O)C3=CC(=C(C(=C3)O)O)O)OC(=O)C4=CC(=C(C(=C4)O)O)O)OC(=O)C5=CC(=C(C(=C5)O)O)O)OC(=O)C6=CC(=C(C(=C6)O)O)O
InChI : InChI=1S\/C41H32O26\/c42-17-1-12(2-18(43)28(17)52)36(57)62-11-27-33(64-37(58)13-3-19(44)29(53)20(45)4-13)34(65-38(59)14-5-21(46)30(54)22(47)6-14)35(66-39(60)15-7-23(48)31(55)24(49)8-15)41(63-27)67-40(61)16-9-25(50)32(56)26(51)10-16\/h1-10,27,33-35,41-56H,11H2\/t27-,33-,34+,35-,41+\/m1\/s1
InChIKey : QJYNZEYHSMRWBK-NIKIMHBISA-N
Other name(s) : PGG, 1,2,3,4,6-penta-O-galloyl-beta-D-glucopyranose, Pentagalloyl glucose, 1,2,3,4,6-Pentagalloylglucose
Target
Families : Plant_carboxylesterase
References (4)
| Title : New insights into the function of plant tannase with promiscuous acyltransferase activity - Chen_2022_Plant.J__ |
| Author(s) : Chen Y , Jiang C , Yin S , Zhuang J , Zhao Y , Zhang L , Jiang X , Liu Y , Gao L , Xia T |
| Ref : Plant J , : , 2022 |
| Abstract : Chen_2022_Plant.J__ |
| ESTHER : Chen_2022_Plant.J__ |
| PubMedSearch : Chen_2022_Plant.J__ |
| PubMedID: 36534122 |
| Gene_locus related to this paper: camsi-CsTA |
| Title : Genome-Wide Identification of Tannase Genes and Their Function of Wound Response and Astringent Substances Accumulation in Juglandaceae - Wang_2021_Front.Plant.Sci_12_664470 |
| Author(s) : Wang J , Wang K , Lyu S , Huang J , Huang C , Xing Y , Wang Y , Xu Y , Li P , Hong J , Xi J , Si X , Ye H , Li Y |
| Ref : Front Plant Sci , 12 :664470 , 2021 |
| Abstract : Wang_2021_Front.Plant.Sci_12_664470 |
| ESTHER : Wang_2021_Front.Plant.Sci_12_664470 |
| PubMedSearch : Wang_2021_Front.Plant.Sci_12_664470 |
| PubMedID: 34079571 |
| Gene_locus related to this paper: camsi-CsTA |
| Title : Discovery and characterization of tannase genes in plants: roles in hydrolysis of tannins - Dai_2020_New.Phytol_226_1104 |
| Author(s) : Dai X , Liu Y , Zhuang J , Yao S , Liu L , Jiang X , Zhou K , Wang Y , Xie D , Bennetzen JL , Gao L , Xia T |
| Ref : New Phytol , 226 :1104 , 2020 |
| Abstract : Dai_2020_New.Phytol_226_1104 |
| ESTHER : Dai_2020_New.Phytol_226_1104 |
| PubMedSearch : Dai_2020_New.Phytol_226_1104 |
| PubMedID: 32061142 |
| Gene_locus related to this paper: camsi-a0a5b9g8c2 , jugre-JrTA , fraan-FaTA , vitvi-VvTA , citcl-CcTA , dioka-DkTA , camsi-CsTA |
| Title : Tannin acyl hydrolase (EC 3.1.1.20) activity of Aspergillus, Penicillium, Fusarium and Trichoderma - Bajpai_1996_World.J.Microbiol.Biotechnol_12_217 |
| Author(s) : Bajpai B , Patil S |
| Ref : World J Microbiol Biotechnol , 12 :217 , 1996 |
| Abstract : Bajpai_1996_World.J.Microbiol.Biotechnol_12_217 |
| ESTHER : Bajpai_1996_World.J.Microbiol.Biotechnol_12_217 |
| PubMedSearch : Bajpai_1996_World.J.Microbiol.Biotechnol_12_217 |
| PubMedID: 24415228 |