TSO
General
Type : Epoxide
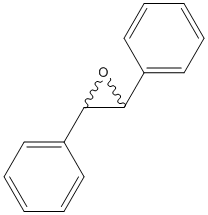
Chemical_Nomenclature : (2S,3S)-2,3-diphenyloxirane
Canonical SMILES : C1=CC=C(C=C1)C2C(O2)C3=CC=CC=C3
InChI : InChI=1S\/C14H12O\/c1-3-7-11(8-4-1)13-14(15-13)12-9-5-2-6-10-12\/h1-10,13-14H\/t13-,14-\/m0\/s1
InChIKey : ARCJQKUWGAZPFX-KBPBESRZSA-N
Other name(s) : Trans-Stilbene oxide, t-SO, Trans-1,2-Diphenyloxirane, (S,S)-stilbene oxide, Trans-2,3-Diphenyloxirane, UNII-D2L0AGD7AK, Trans-alpha,alpha-Epoxydibenzyl
MW : 196.24
Formula : C14H12O
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Epoxide_hydrolase
References (5)
| Title : Substrate and inhibitor selectivity, and biological activity of an epoxide hydrolase from Trichoderma reesei - de Oliveira_2019_Mol.Biol.Rep_46_371 |
| Author(s) : de Oliveira GS , Adriani PP , Wu H , Morisseau C , Hammock BD , Chambergo FS |
| Ref : Mol Biol Rep , 46 :371 , 2019 |
| Abstract : de Oliveira_2019_Mol.Biol.Rep_46_371 |
| ESTHER : de Oliveira_2019_Mol.Biol.Rep_46_371 |
| PubMedSearch : de Oliveira_2019_Mol.Biol.Rep_46_371 |
| PubMedID: 30426381 |
| Gene_locus related to this paper: hypjq-g0r7e2 |
| Title : Epoxide hydrolase-catalyzed enantioselective conversion of trans-stilbene oxide: Insights into the reaction mechanism from steady-state and pre-steady-state enzyme kinetics - Archelas_2016_Arch.Biochem.Biophys_591_66 |
| Author(s) : Archelas A , Zhao W , Faure B , Iacazio G , Kotik M |
| Ref : Archives of Biochemistry & Biophysics , 591 :66 , 2016 |
| Abstract : Archelas_2016_Arch.Biochem.Biophys_591_66 |
| ESTHER : Archelas_2016_Arch.Biochem.Biophys_591_66 |
| PubMedSearch : Archelas_2016_Arch.Biochem.Biophys_591_66 |
| PubMedID: 26714303 |
| Title : Expanding the Catalytic Triad in Epoxide Hydrolases and Related Enzymes - Amrein_2015_ACS.Catal_5_5702 |
| Author(s) : Amrein BA , Bauer P , Duarte F , Carlsson AJ , Naworyta A , Mowbray SL , Widersten M , Kamerlin SCL |
| Ref : ACS Catal , 5 :5702 , 2015 |
| Abstract : Amrein_2015_ACS.Catal_5_5702 |
| ESTHER : Amrein_2015_ACS.Catal_5_5702 |
| PubMedSearch : Amrein_2015_ACS.Catal_5_5702 |
| PubMedID: 26527505 |
| Gene_locus related to this paper: soltu-3epoxy |
| Title : Implications for an ionized alkyl-enzyme intermediate during StEH1-catalyzed trans-stilbene oxide hydrolysis - Elfstrom_2006_Biochemistry_45_205 |
| Author(s) : Elfstrom LT , Widersten M |
| Ref : Biochemistry , 45 :205 , 2006 |
| Abstract : Elfstrom_2006_Biochemistry_45_205 |
| ESTHER : Elfstrom_2006_Biochemistry_45_205 |
| PubMedSearch : Elfstrom_2006_Biochemistry_45_205 |
| PubMedID: 16388596 |
| Gene_locus related to this paper: soltu-3epoxy |
| Title : The effect of tridiphane (2-(3,5-dichlorophenyl)-2-(2,2,2-trichloroethyl)oxirane) on hepatic epoxide-metabolizing enzymes: indications of peroxisome proliferation - Moody_1987_Toxicol.Appl.Pharmacol_89_37 |
| Author(s) : Moody DE , Hammock BD |
| Ref : Toxicol Appl Pharmacol , 89 :37 , 1987 |
| Abstract : Moody_1987_Toxicol.Appl.Pharmacol_89_37 |
| ESTHER : Moody_1987_Toxicol.Appl.Pharmacol_89_37 |
| PubMedSearch : Moody_1987_Toxicol.Appl.Pharmacol_89_37 |
| PubMedID: 3590187 |