Benzyl-2-triazole-linked-tryptamine-paeonol-Cpd896
896 non-competitive inhibitor BChE with an IC50 value of 0.13 microM. IC50 AChE > 100 microM; IC50 BChE 0.13 +/- 0.05; IC50 MAO-A 54.02 +/- 4.11 microM; IC50 MAO-B 31.78 +/- 1.73 microM
General
Type : Triazol, Trifluoro, Derivative of Tryptamine, Indole
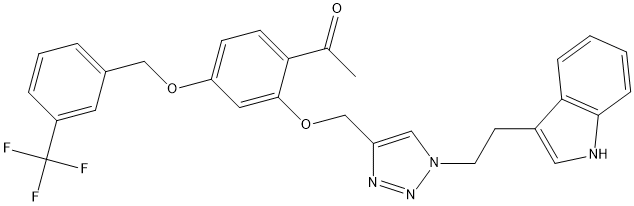
Chemical_Nomenclature : 1-(2-((1-(2-(1H-indol-3-yl)ethyl)-1H-1,2,3-triazol-4-yl)methoxy)-4-((3-(trifluoromethyl)benzyl)oxy)phenyl)ethanone
Canonical SMILES : C1=CC(=CC(=C1)COC2=CC=C(C(=C2)OCC3=C[N](N=N3)CCC4=C[N]C5=C4C=CC=C5)C(=O)C)C(F)(F)F
InChI : InChI=1S\/C29H25F3N4O3\/c1-19(37)25-10-9-24(38-17-20-5-4-6-22(13-20)29(30,31)32)14-28(25)39-18-23-16-36(35-34-23)12-11-21-15-33-27-8-3-2-7-26(21)27\/h2-10,13-16,33H,11-12,17-18H2,1H3
InChIKey : BDVQWVIBCQHBCG-UHFFFAOYSA-N
Other name(s) :
Target
Families : Benzyl-2-triazole-linked-tryptamine-paeonol-Cpd896 ligand of proteins in family
BCHE
Protein :
human-BCHE
References (1)
| Title : Synthesis of 4-substituted benzyl-2-triazole-linked-tryptamine-paeonol derivatives and evaluation of their selective inhibitions against butyrylcholinesterase and monoamine oxidase-B - Oh_2022_Int.J.Biol.Macromol_217_910 |
| Author(s) : Oh JM , Kang Y , Hwang JH , Park JH , Shin WH , Mun SK , Lee JU , Yee ST , Kim H |
| Ref : Int J Biol Macromol , 217 :910 , 2022 |
| Abstract : |
| PubMedSearch : Oh_2022_Int.J.Biol.Macromol_217_910 |
| PubMedID: 35908673 |