CHEMBL4292235
Compound 9a acted as a potent, selective and bifunctional AChEI (IC50=0.21muM, Ki=0.19muM) and displayed dual hMAO inhibitory activity (hMAO-A IC50=0.94muM, Ki=0.057muM and hMAO-B IC50=3.81muM, Ki=0.48muM).
General
Type : Multitarget, Monoamine-oxidase-inhibitor, Chromen
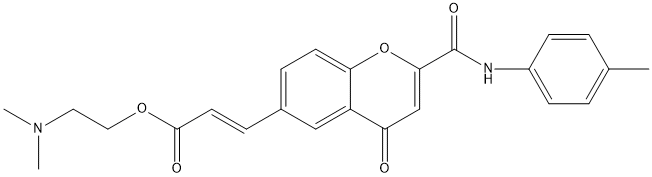
Chemical_Nomenclature : 2-(dimethylamino)ethyl (E)-3-[2-[(4-methylphenyl)carbamoyl]-4-oxochromen-6-yl]prop-2-enoate
Canonical SMILES : CC1=CC=C(C=C1)NC(=O)C2=CC(=O)C3=C(O2)C=CC(=C3)C=CC(=O)OCCN(C)C
InChI : InChI=1S\/C24H24N2O5\/c1-16-4-8-18(9-5-16)25-24(29)22-15-20(27)19-14-17(6-10-21(19)31-22)7-11-23(28)30-13-12-26(2)3\/h4-11,14-15H,12-13H2,1-3H3,(H,25,29)\/b11-7+
InChIKey : JRQGALQTHYTIML-YRNVUSSQSA-N
Other name(s) : 2-(Dimethylamino)ethyl (E)-3-(4-oxo-2-((4-methylphenyl)carbamoyl)-4H-chromen-6-yl)acrylate || Compound 9a || CHEMBL4292235 || BDBM50467507
MW : 420.5
Formula : C24H25N2O5
CAS_number :
PubChem : 145988532
UniChem : JRQGALQTHYTIML-YRNVUSSQSA-N
Target
Families : CHEMBL4292235 ligand of proteins in family
ACHE
Protein :
human-ACHE
References (1)
| Title : Multi-target-directed ligands for Alzheimer's disease: Discovery of chromone-based monoamine oxidase\/cholinesterase inhibitors - Reis_2018_Eur.J.Med.Chem_158_781 |
| Author(s) : Reis J , Cagide F , Valencia ME , Teixeira J , Bagetta D , Perez C , Uriarte E , Oliveira PJ , Ortuso F , Alcaro S , Rodriguez-Franco MI , Borges F |
| Ref : Eur Journal of Medicinal Chemistry , 158 :781 , 2018 |
| Abstract : |
| PubMedSearch : Reis_2018_Eur.J.Med.Chem_158_781 |
| PubMedID: 30245401 |