DLI
General
Type : Trifluoro, Sulfur Compound, Pyrrolidine, Pyrimidine
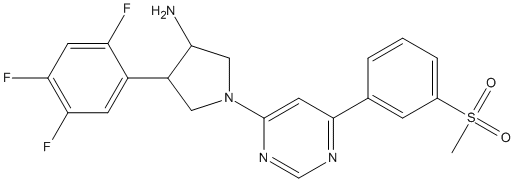
Chemical_Nomenclature : (3R,4S)-1-[6-(3-methylsulfonylphenyl)pyrimidin-4-yl]-4-(2,4,5-trifluorophenyl)pyrrolidin-3-amine
Canonical SMILES : CS(=O)(=O)C1=CC=CC(=C1)C2=CC(=NC=N2)N3CC(C(C3)N)C4=CC(=C(C=C4F)F)F
InChI : InChI=1S\/C21H19F3N4O2S\/c1-31(29,30)13-4-2-3-12(5-13)20-8-21(27-11-26-20)28-9-15(19(25)10-28)14-6-17(23)18(24)7-16(14)22\/h2-8,11,15,19H,9-10,25H2,1H3\/t15-,19+\/m1\/s1
InChIKey : OAWGQHXWGXOUKV-BEFAXECRSA-N
Other name(s) : (3r,4s)-1-{6-[3-(Methylsulfonyl)phenyl]pyrimidin-4-Yl}-4-(2,4,5-Trifluorophenyl)pyrrolidin-3-Amine || CHEMBL1232261 || BDBM15498 || DB07666 || Pyrrolidine-constrained phenethylamine 29g
MW : 448.46
Formula : C21H19F3N4O2S
CAS_number :
PubChem : 16750059
UniChem : OAWGQHXWGXOUKV-BEFAXECRSA-N
Target
Families : DLI ligand of proteins in family
DPP4N_Peptidase_S9
Structure :
2OAG
Protein :
human-DPP4
References (3)
| Title : Pyrrolidine-constrained phenethylamines: The design of potent, selective, and pharmacologically efficacious dipeptidyl peptidase IV (DPP4) inhibitors from a lead-like screening hit - Backes_2007_Bioorg.Med.Chem.Lett_17_2005 |
| Author(s) : Backes BJ , Longenecker K , Hamilton GL , Stewart K , Lai C , Kopecka H , von Geldern TW , Madar DJ , Pei Z , Lubben TH , Zinker BA , Tian Z , Ballaron SJ , Stashko MA , Mika AK , Beno DW , Kempf-Grote AJ , Black-Schaefer C , Sham HL , Trevillyan JM |
| Ref : Bioorganic & Medicinal Chemistry Lett , 17 :2005 , 2007 |
| Abstract : |
| PubMedSearch : Backes_2007_Bioorg.Med.Chem.Lett_17_2005 |
| PubMedID: 17276063 |
| Gene_locus related to this paper: ratno-dpp4 , human-DPP4 |
| Title : Pyrrolidine-constrained phenethylamines: The design of potent, selective, and pharmacologically efficacious dipeptidyl peptidase IV (DPP4) inhibitors from a lead-like screening hit - Backes_2007_Bioorg.Med.Chem.Lett_17_2005 |
| Author(s) : Backes BJ , Longenecker K , Hamilton GL , Stewart K , Lai C , Kopecka H , von Geldern TW , Madar DJ , Pei Z , Lubben TH , Zinker BA , Tian Z , Ballaron SJ , Stashko MA , Mika AK , Beno DW , Kempf-Grote AJ , Black-Schaefer C , Sham HL , Trevillyan JM |
| Ref : Bioorganic & Medicinal Chemistry Lett , 17 :2005 , 2007 |
| Abstract : |
| PubMedSearch : Backes_2007_Bioorg.Med.Chem.Lett_17_2005 |
| PubMedID: 17276063 |
| Gene_locus related to this paper: ratno-dpp4 , human-DPP4 |
| Title : Pyrrolidine-constrained phenethylamines: The design of potent, selective, and pharmacologically efficacious dipeptidyl peptidase IV (DPP4) inhibitors from a lead-like screening hit - Backes_2007_Bioorg.Med.Chem.Lett_17_2005 |
| Author(s) : Backes BJ , Longenecker K , Hamilton GL , Stewart K , Lai C , Kopecka H , von Geldern TW , Madar DJ , Pei Z , Lubben TH , Zinker BA , Tian Z , Ballaron SJ , Stashko MA , Mika AK , Beno DW , Kempf-Grote AJ , Black-Schaefer C , Sham HL , Trevillyan JM |
| Ref : Bioorganic & Medicinal Chemistry Lett , 17 :2005 , 2007 |
| Abstract : |
| PubMedSearch : Backes_2007_Bioorg.Med.Chem.Lett_17_2005 |
| PubMedID: 17276063 |
| Gene_locus related to this paper: ratno-dpp4 , human-DPP4 |