HDAC6-IN-5
Combined Givinostat inhibitor of HDAC with Tacrine. Potent HDAC6 inhibitor (IC50 26nM); anti abeta aggregation (IC50 1.1 microM); strong Abeta disaggregation 70%; IC50 AChE 0.88 microM
General
Type : Multitarget, HDAC inhibitor, Derivative of Tacrine, anti-Abeta-aggregation
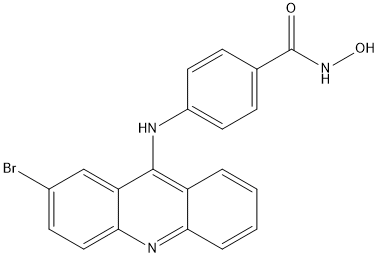
Chemical_Nomenclature : 4-[(2-bromoacridin-9-yl)amino]-N-hydroxybenzamide
Canonical SMILES : C1=CC=C2C(=C1)C(=C3C=C(C=CC3=N2)Br)NC4=CC=C(C=C4)C(=O)NO
InChI : InChI=1S\/C20H14BrN3O2\/c21-13-7-10-18-16(11-13)19(15-3-1-2-4-17(15)23-18)22-14-8-5-12(6-9-14)20(25)24-26\/h1-11,26H,(H,22,23)(H,24,25)
InChIKey : PESSDQDERPAVNH-UHFFFAOYSA-N
Other name(s) : CHEMBL4796066 || BDBM50551544 || HY-146678 || CS-0438309
MW : 408.2
Formula : C20H14BrN3O2
CAS_number : 2413603-15-1
PubChem : 162673723
UniChem : PESSDQDERPAVNH-UHFFFAOYSA-N
Target
Families : HDAC6-IN-5 ligand of proteins in family
ACHE
Protein :
human-ACHE
References (1)
| Title : Synthesis and biological evaluation of acridine-based histone deacetylase inhibitors as multitarget agents against Alzheimer's disease - Tseng_2020_Eur.J.Med.Chem_192_112193 |
| Author(s) : Tseng HJ , Lin MH , Shiao YJ , Yang YC , Chu JC , Chen CY , Chen YY , Lin TE , Su CJ , Pan SL , Chen LC , Wang CY , Hsu KC , Huang WJ |
| Ref : Eur Journal of Medicinal Chemistry , 192 :112193 , 2020 |
| Abstract : |
| PubMedSearch : Tseng_2020_Eur.J.Med.Chem_192_112193 |
| PubMedID: 32151835 |