MAGL17b
Ki = 0.65 microM IC50=0.84 microM
General
Type : Piperidine, Organochlorine
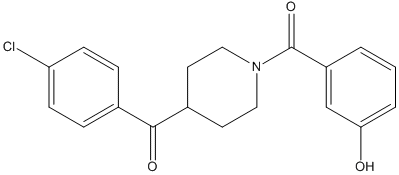
Chemical_Nomenclature : (4-chlorophenyl)-[1-(3-hydroxybenzoyl)piperidin-4-yl]methanone
Canonical SMILES : C1CN(CCC1C(=O)C2=CC=C(C=C2)Cl)C(=O)C3=CC(=CC=C3)O
InChI : InChI=1S\/C19H18ClNO3\/c20-16-6-4-13(5-7-16)18(23)14-8-10-21(11-9-14)19(24)15-2-1-3-17(22)12-15\/h1-7,12,14,22H,8-11H2
InChIKey : XSAQFVZJTOJQCI-UHFFFAOYSA-N
Other name(s) : CHEMBL4282040 || BDBM50470454 || J3.612.244G || 1-(3-Hydroxybenzoyl)-4-(4-chlorobenzoyl)piperidine
MW : 343.8
Formula : C19H18ClNO3
CAS_number :
PubChem : 132941103
UniChem : XSAQFVZJTOJQCI-UHFFFAOYSA-N
Target
Families : MAGL17b ligand of proteins in family
Monoglyceridelipase_lysophospholip
Protein :
human-MGLL
References (6)
| Title : MAGL inhibitor NanoMicellar formulation (MAGL-NanoMicellar) for the development of an antiglaucoma eye drop - Chetoni_2022_Int.J.Pharm__122078 |
| Author(s) : Chetoni P , Burgalassi S , Zucchetti E , Granchi C , Minutolo F , Tampucci S , Monti D |
| Ref : Int J Pharm , :122078 , 2022 |
| Abstract : |
| PubMedSearch : Chetoni_2022_Int.J.Pharm__122078 |
| PubMedID: 35932931 |
| Gene_locus related to this paper: human-MGLL |
| Title : MAGL inhibitor NanoMicellar formulation (MAGL-NanoMicellar) for the development of an antiglaucoma eye drop - Chetoni_2022_Int.J.Pharm__122078 |
| Author(s) : Chetoni P , Burgalassi S , Zucchetti E , Granchi C , Minutolo F , Tampucci S , Monti D |
| Ref : Int J Pharm , :122078 , 2022 |
| Abstract : |
| PubMedSearch : Chetoni_2022_Int.J.Pharm__122078 |
| PubMedID: 35932931 |
| Gene_locus related to this paper: human-MGLL |
| Title : MAGL inhibitor NanoMicellar formulation (MAGL-NanoMicellar) for the development of an antiglaucoma eye drop - Chetoni_2022_Int.J.Pharm__122078 |
| Author(s) : Chetoni P , Burgalassi S , Zucchetti E , Granchi C , Minutolo F , Tampucci S , Monti D |
| Ref : Int J Pharm , :122078 , 2022 |
| Abstract : |
| PubMedSearch : Chetoni_2022_Int.J.Pharm__122078 |
| PubMedID: 35932931 |
| Gene_locus related to this paper: human-MGLL |
| Title : Structural Optimization of 4-Chlorobenzoylpiperidine Derivatives for the Development of Potent, Reversible, and Selective Monoacylglycerol Lipase (MAGL) Inhibitors - Granchi_2016_J.Med.Chem_59_10299 |
| Author(s) : Granchi C , Rizzolio F , Palazzolo S , Carmignani S , Macchia M , Saccomanni G , Manera C , Martinelli A , Minutolo F , Tuccinardi T |
| Ref : Journal of Medicinal Chemistry , 59 :10299 , 2016 |
| Abstract : |
| PubMedSearch : Granchi_2016_J.Med.Chem_59_10299 |
| PubMedID: 27809504 |
| Title : Structural Optimization of 4-Chlorobenzoylpiperidine Derivatives for the Development of Potent, Reversible, and Selective Monoacylglycerol Lipase (MAGL) Inhibitors - Granchi_2016_J.Med.Chem_59_10299 |
| Author(s) : Granchi C , Rizzolio F , Palazzolo S , Carmignani S , Macchia M , Saccomanni G , Manera C , Martinelli A , Minutolo F , Tuccinardi T |
| Ref : Journal of Medicinal Chemistry , 59 :10299 , 2016 |
| Abstract : |
| PubMedSearch : Granchi_2016_J.Med.Chem_59_10299 |
| PubMedID: 27809504 |
| Title : Structural Optimization of 4-Chlorobenzoylpiperidine Derivatives for the Development of Potent, Reversible, and Selective Monoacylglycerol Lipase (MAGL) Inhibitors - Granchi_2016_J.Med.Chem_59_10299 |
| Author(s) : Granchi C , Rizzolio F , Palazzolo S , Carmignani S , Macchia M , Saccomanni G , Manera C , Martinelli A , Minutolo F , Tuccinardi T |
| Ref : Journal of Medicinal Chemistry , 59 :10299 , 2016 |
| Abstract : |
| PubMedSearch : Granchi_2016_J.Med.Chem_59_10299 |
| PubMedID: 27809504 |