Cinchonine
Cinchonine and Cinchonidine are natural products found in Dendrosenecio kilimanjari, Cinchona calisaya, CD Ki BCHE 28+/-4 Micro M Ki ACHE >400 micro M; CN Ki BCHE 4.9 +/- 1.4 microM Ki ACHE 34 +/- 1 microM. Poor inhibitor but lead for new activr compounds
General
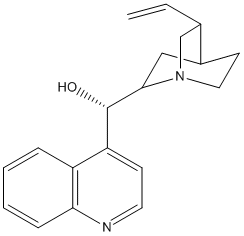
Type : Natural,Alkaloid,Derivative of Cinchonine,Quinoline,Azabicyclo
Chemical_Nomenclature : (5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl)-quinolin-4-ylmethanol
Canonical SMILES : C=CC1CN2CCC1CC2C(C3=CC=NC4=CC=CC=C34)O
InChI : InChI=1S\/C19H22N2O\/c1-2-13-12-21-10-8-14(13)11-18(21)19(22)16-7-9-20-17-6-4-3-5-15(16)17\/h2-7,9,13-14,18-19,22H,1,8,10-12H2
InChIKey : KMPWYEUPVWOPIM-UHFFFAOYSA-N
Other name(s) : Cinchonan-9-ol,alpha-Quinidine
MW : 294.4
Formula : C19H22N2O
CAS_number : 118-10-5 || 485-71-2
PubChem : 2757
UniChem : KMPWYEUPVWOPIM-UHFFFAOYSA-N
Target
Families : Cinchonine ligand of proteins in family
BCHE
References (4)
| Title : Design and evaluation of selective butyrylcholinesterase inhibitors based on Cinchona alkaloid scaffold - Bosak_2018_PLoS.One_13_e0205193 |
| Author(s) : Bosak A , Ramic A , Smidlehner T , Hrenar T , Primozic I , Kovarik Z |
| Ref : PLoS ONE , 13 :e0205193 , 2018 |
| Abstract : Bosak_2018_PLoS.One_13_e0205193 |
| ESTHER : Bosak_2018_PLoS.One_13_e0205193 |
| PubMedSearch : Bosak_2018_PLoS.One_13_e0205193 |
| PubMedID: 30289893 |
| Title : New Cinchona Oximes Evaluated as Reactivators of Acetylcholinesterase and Butyrylcholinesterase Inhibited by Organophosphorus Compounds - Katalinic_2017_Molecules_22_ |
| Author(s) : Katalinic M , Zandona A , Ramic A , Zorbaz T , Primozic I , Kovarik Z |
| Ref : Molecules , 22 : , 2017 |
| Abstract : Katalinic_2017_Molecules_22_ |
| ESTHER : Katalinic_2017_Molecules_22_ |
| PubMedSearch : Katalinic_2017_Molecules_22_ |
| PubMedID: 28737687 |
| Title : Chemical modifications of cinchona alkaloids lead to enhanced inhibition of human butyrylcholinesterase - Karlsson_2014_Nat.Prod.Commun_9_455 |
| Author(s) : Karlsson D , Fallarero A , Shinde P , Anju CP , Busygin I , Leino R , Mohan CG , Vuorela P |
| Ref : Nat Prod Commun , 9 :455 , 2014 |
| Abstract : Karlsson_2014_Nat.Prod.Commun_9_455 |
| ESTHER : Karlsson_2014_Nat.Prod.Commun_9_455 |
| PubMedSearch : Karlsson_2014_Nat.Prod.Commun_9_455 |
| PubMedID: 24868853 |
| Title : Cation-Pi and Pi-Pi stacking interactions allow selective inhibition of butyrylcholinesterase by modified quinine and cinchonidine alkaloids - Nawaz_2011_Biochem.Biophys.Res.Commun_404_935 |
| Author(s) : Nawaz SA , Ayaz M , Brandt W , Wessjohann LA , Westermann B |
| Ref : Biochemical & Biophysical Research Communications , 404 :935 , 2011 |
| Abstract : Nawaz_2011_Biochem.Biophys.Res.Commun_404_935 |
| ESTHER : Nawaz_2011_Biochem.Biophys.Res.Commun_404_935 |
| PubMedSearch : Nawaz_2011_Biochem.Biophys.Res.Commun_404_935 |
| PubMedID: 21185266 |