Methamidophos
Methamidophos is also a breakdown product of the organophosphate insecticide acephate (Orthene).
General
Type : Insecticide,Organophosphate,Sulfur Compound
Chemical_Nomenclature : [amino(methylsulfanyl)phosphoryl]oxymethane
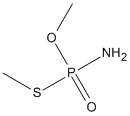
Canonical SMILES : COP(=O)(N)SC
InChI : InChI=1S\/C2H8NO2PS\/c1-5-6(3,4)7-2\/h1-2H3,(H2,3,4)
InChIKey : NNKVPIKMPCQWCG-UHFFFAOYSA-N
Other name(s) : O,S-Dimethylphosphora-midothiolate,Phosphoramidothioic acid O,S-dimethyl ester,Metamidophos,Pillaron,Tahmabon,Filitox,Hamidop,Patrole,Tamanox,Tamaron,Amidor,Product names include Monitor, Nitofol, Tamaron, Swipe, Nuratron, Vetaron, Filitox, Patrole, Tamanox, SRA 5172, and Tam.
MW : 141.12
Formula : C2H8NO2PS
CAS_number : 10265-92-6
PubChem : 4096
UniChem : NNKVPIKMPCQWCG-UHFFFAOYSA-N
IUPHAR :
Wikipedia : Methamidophos
Target
References (12)
| Title : Organophosphorus pesticide-induced butyrylcholinesterase inhibition and potentiation of succinylcholine toxicity in mice - Sparks_1999_J.Biochem.Mol.Toxicol_13_113 |
| Author(s) : Sparks SE , Quistad GB , Casida JE |
| Ref : J Biochem Mol Toxicol , 13 :113 , 1999 |
| Abstract : Sparks_1999_J.Biochem.Mol.Toxicol_13_113 |
| ESTHER : Sparks_1999_J.Biochem.Mol.Toxicol_13_113 |
| PubMedSearch : Sparks_1999_J.Biochem.Mol.Toxicol_13_113 |
| PubMedID: 9890196 |
| Title : Physicochemical, molecular-orbital and electronic properties of acephate and methamidophos - Singh_1998_Comp.Biochem.Physiol.C.Pharmacol.Toxicol.Endocrinol_119_107 |
| Author(s) : Singh AK , White T , Spassova D , Jiang Y |
| Ref : Comparative Biochemistry & Physiology C Pharmacology Toxicology & Endocrinology , 119 :107 , 1998 |
| Abstract : Singh_1998_Comp.Biochem.Physiol.C.Pharmacol.Toxicol.Endocrinol_119_107 |
| ESTHER : Singh_1998_Comp.Biochem.Physiol.C.Pharmacol.Toxicol.Endocrinol_119_107 |
| PubMedSearch : Singh_1998_Comp.Biochem.Physiol.C.Pharmacol.Toxicol.Endocrinol_119_107 |
| PubMedID: 9568380 |
| Title : Protective effect of pralidoxime on muscle fiber necrosis induced by organophosphate compounds - Cavaliere_1998_J.Toxicol.Clin.Toxicol_36_295 |
| Author(s) : Cavaliere MJ , Puga FR , Calore EE , Calore NM , Pelegrino JR , da Rosa AR , Weg R |
| Ref : Journal of Toxicology Clinical Toxicology , 36 :295 , 1998 |
| Abstract : Cavaliere_1998_J.Toxicol.Clin.Toxicol_36_295 |
| ESTHER : Cavaliere_1998_J.Toxicol.Clin.Toxicol_36_295 |
| PubMedSearch : Cavaliere_1998_J.Toxicol.Clin.Toxicol_36_295 |
| PubMedID: 9711194 |
| Title : Oxidative bioactivation of methamidophos insecticide: synthesis of N- hydroxymethamidophos (a candidate metabolite) and its proposed alternative reactions involving N-->O rearrangement or fragmentation through a metaphosphate analogue - Mahajna_1998_Chem.Res.Toxicol_11_26 |
| Author(s) : Mahajna M , Casida JE |
| Ref : Chemical Research in Toxicology , 11 :26 , 1998 |
| Abstract : Mahajna_1998_Chem.Res.Toxicol_11_26 |
| ESTHER : Mahajna_1998_Chem.Res.Toxicol_11_26 |
| PubMedSearch : Mahajna_1998_Chem.Res.Toxicol_11_26 |
| PubMedID: 9477223 |
| Title : Risk factors for systemic illnesses following agricultural exposures to restricted organophosphates in California, 1984-1988 - Weinbaum_1997_Am.J.Ind.Med_31_572 |
| Author(s) : Weinbaum Z , Schenker MB , Gold EB , Samuels SJ , O'Malley MA |
| Ref : American Journal of Industrial Medicine , 31 :572 , 1997 |
| Abstract : Weinbaum_1997_Am.J.Ind.Med_31_572 |
| ESTHER : Weinbaum_1997_Am.J.Ind.Med_31_572 |
| PubMedSearch : Weinbaum_1997_Am.J.Ind.Med_31_572 |
| PubMedID: 9099360 |
| Title : Intravenous organophosphate injection: an unusual way of intoxication - Guven_1997_Hum.Exp.Toxicol_16_279 |
| Author(s) : Guven M , Unluhizarci K , Goktas Z , Kurtoglu S |
| Ref : Hum Exp Toxicol , 16 :279 , 1997 |
| Abstract : Guven_1997_Hum.Exp.Toxicol_16_279 |
| ESTHER : Guven_1997_Hum.Exp.Toxicol_16_279 |
| PubMedSearch : Guven_1997_Hum.Exp.Toxicol_16_279 |
| PubMedID: 9192209 |
| Title : Subchronic neurotoxicity screening studies with six organophosphate insecticides: an assessment of behavior and morphology relative to cholinesterase inhibition - Sheets_1997_Fundam.Appl.Toxicol_35_101 |
| Author(s) : Sheets LP , Hamilton BF , Sangha GK , Thyssen JH |
| Ref : Fundamental & Applied Toxicology , 35 :101 , 1997 |
| Abstract : Sheets_1997_Fundam.Appl.Toxicol_35_101 |
| ESTHER : Sheets_1997_Fundam.Appl.Toxicol_35_101 |
| PubMedSearch : Sheets_1997_Fundam.Appl.Toxicol_35_101 |
| PubMedID: 9024678 |
| Title : Residues and half-lives of acephate, methamidophos, and pirimiphos- methyl in leaves and fruit of greenhouse-grown tomatoes - |
| Author(s) : Antonious GF , Snyder JC |
| Ref : Bulletin of Environmental Contamination & Toxicology , 52 :141 , 1994 |
| PubMedID: 8130409 |
| Title : Molecular properties and inhibition kinetics of acetylcholinesterase obtained from rat brain and cockroach ganglion - Singh_1990_Toxicol.Ind.Health_6_551 |
| Author(s) : Singh AK |
| Ref : Toxicol Ind Health , 6 :551 , 1990 |
| Abstract : Singh_1990_Toxicol.Ind.Health_6_551 |
| ESTHER : Singh_1990_Toxicol.Ind.Health_6_551 |
| PubMedSearch : Singh_1990_Toxicol.Ind.Health_6_551 |
| PubMedID: 2097819 |
| Title : [Exposure to residues on plant surfaces following the use of plant pesticides in the greenhouse] - Goedicke_1989_Z.Gesamte.Hyg_35_531 |
| Author(s) : Goedicke HJ , Hermes H , Wagner R |
| Ref : Z Gesamte Hyg , 35 :531 , 1989 |
| Abstract : Goedicke_1989_Z.Gesamte.Hyg_35_531 |
| ESTHER : Goedicke_1989_Z.Gesamte.Hyg_35_531 |
| PubMedSearch : Goedicke_1989_Z.Gesamte.Hyg_35_531 |
| PubMedID: 2588705 |
| Title : Studies on the toxicity, metabolism, and anticholinesterase properties of acephate and methamidophos - Hussain_1985_J.Env.Sci.Health_20_129 |
| Author(s) : Hussain MA , Mohamad RB , Oloffs PC |
| Ref : Journal of Environmental Science & Health Part B: Pesticides, Food Contaminants, & Agricultural Wastes , 20 :129 , 1985 |
| Abstract : Hussain_1985_J.Env.Sci.Health_20_129 |
| ESTHER : Hussain_1985_J.Env.Sci.Health_20_129 |
| PubMedSearch : Hussain_1985_J.Env.Sci.Health_20_129 |
| PubMedID: 3989221 |
| Title : Inhibition of human erythrocyte and plasma cholinesterases by methamidophos - Robinson_1982_J.Appl.Toxicol_2_217 |
| Author(s) : Robinson CP , Beiergrohslein D |
| Ref : Journal of Applied Toxicology , 2 :217 , 1982 |
| Abstract : Robinson_1982_J.Appl.Toxicol_2_217 |
| ESTHER : Robinson_1982_J.Appl.Toxicol_2_217 |
| PubMedSearch : Robinson_1982_J.Appl.Toxicol_2_217 |
| PubMedID: 7185904 |