K108
General
Type : Oxime, Bisoxime, Bispyridinium
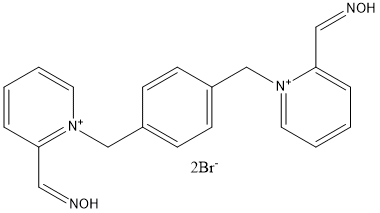
Chemical_Nomenclature : (NE)-N-[[1-[[4-[[2-[(E)-hydroxyiminomethyl]pyridin-1-ium-1-yl]methyl]phenyl]methyl]pyridin-1-ium-2-yl]methylidene]hydroxylamine\;dibromide
Canonical SMILES : C1=CC=[N+](C(=C1)\/C=N\/O)CC2=CC=C(C=C2)C[N+]3=CC=CC=C3\/C=N\/O.[Br-].[Br-]
InChI : InChI=1S\/C20H18N4O2.2BrH\/c25-21-13-19-5-1-3-11-23(19)15-17-7-9-18(10-8-17)16-24-12-4-2-6-20(24)14-22-26\;\;\/h1-14H,15-16H2\;2*1H
InChIKey : FVABVHZFLIGSRE-UHFFFAOYSA-N
Other name(s) : CHEMBL490729
MW : 508.2
Formula : C20H20Br2N4O2
CAS_number :
PubChem : 135871403, 135871404
UniChem : FVABVHZFLIGSRE-UHFFFAOYSA-N
Target
Structure : No structure
Families : No family
References (6)
| Title : Monooxime Bispyridinium Reactivators Bearing Xylene Linker Synthesis and In Vitro Evaluation on Model of Organophosphate-Inhibited Acetylcholinesterase - Musilek_2016_Med.Chem_12_362 |
| Author(s) : Musilek K , Hambalek J , Holas O , Dohnal V , Kuca K |
| Ref : Med Chem , 12 :362 , 2016 |
| Abstract : |
| PubMedSearch : Musilek_2016_Med.Chem_12_362 |
| PubMedID: 26427931 |
| Title : Monooxime Bispyridinium Reactivators Bearing Xylene Linker Synthesis and In Vitro Evaluation on Model of Organophosphate-Inhibited Acetylcholinesterase - Musilek_2016_Med.Chem_12_362 |
| Author(s) : Musilek K , Hambalek J , Holas O , Dohnal V , Kuca K |
| Ref : Med Chem , 12 :362 , 2016 |
| Abstract : |
| PubMedSearch : Musilek_2016_Med.Chem_12_362 |
| PubMedID: 26427931 |
| Title : Monooxime Bispyridinium Reactivators Bearing Xylene Linker Synthesis and In Vitro Evaluation on Model of Organophosphate-Inhibited Acetylcholinesterase - Musilek_2016_Med.Chem_12_362 |
| Author(s) : Musilek K , Hambalek J , Holas O , Dohnal V , Kuca K |
| Ref : Med Chem , 12 :362 , 2016 |
| Abstract : |
| PubMedSearch : Musilek_2016_Med.Chem_12_362 |
| PubMedID: 26427931 |
| Title : Synthesis of a novel series of non-symmetrical bispyridinium compounds bearing a xylene linker and evaluation of their reactivation activity against tabun and paraoxon-inhibited acetylcholinesterase - Musilek_2007_J.Enzyme.Inhib.Med.Chem_22_425 |
| Author(s) : Musilek K , Holas O , Kuca K , Jun D , Dohnal V , Dolezal M |
| Ref : J Enzyme Inhib Med Chem , 22 :425 , 2007 |
| Abstract : |
| PubMedSearch : Musilek_2007_J.Enzyme.Inhib.Med.Chem_22_425 |
| PubMedID: 17847708 |
| Title : Synthesis of a novel series of non-symmetrical bispyridinium compounds bearing a xylene linker and evaluation of their reactivation activity against tabun and paraoxon-inhibited acetylcholinesterase - Musilek_2007_J.Enzyme.Inhib.Med.Chem_22_425 |
| Author(s) : Musilek K , Holas O , Kuca K , Jun D , Dohnal V , Dolezal M |
| Ref : J Enzyme Inhib Med Chem , 22 :425 , 2007 |
| Abstract : |
| PubMedSearch : Musilek_2007_J.Enzyme.Inhib.Med.Chem_22_425 |
| PubMedID: 17847708 |
| Title : Synthesis of a novel series of non-symmetrical bispyridinium compounds bearing a xylene linker and evaluation of their reactivation activity against tabun and paraoxon-inhibited acetylcholinesterase - Musilek_2007_J.Enzyme.Inhib.Med.Chem_22_425 |
| Author(s) : Musilek K , Holas O , Kuca K , Jun D , Dohnal V , Dolezal M |
| Ref : J Enzyme Inhib Med Chem , 22 :425 , 2007 |
| Abstract : |
| PubMedSearch : Musilek_2007_J.Enzyme.Inhib.Med.Chem_22_425 |
| PubMedID: 17847708 |