Phenyltetrahydroisoquinoline-Pyridinaldoxime-1c
General
Type : Oxime, Pyridine-aldoxime, Isoquinoline
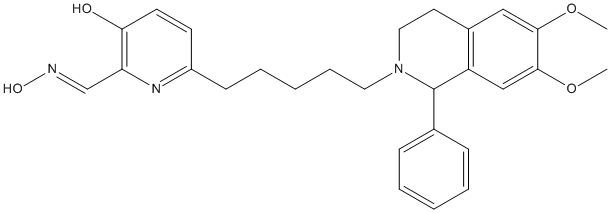
Chemical_Nomenclature : 6-[5-(6,7-dimethoxy-1-phenyl-3,4-dihydro-1H-isoquinolin-2-yl)pentyl]-2-[(E)-hydroxyiminomethyl]pyridin-3-ol
Canonical SMILES : COC1=C(C=C2C(N(CCC2=C1)CCCCCC3=NC(=C(C=C3)O)C=NO)C4=CC=CC=C4)OC
InChI : InChI=1S\/C28H33N3O4\/c1-34-26-17-21-14-16-31(28(20-9-5-3-6-10-20)23(21)18-27(26)35-2)15-8-4-7-11-22-12-13-25(32)24(30-22)19-29-33\/h3,5-6,9-10,12-13,17-19,28,32-33H,4,7-8,11,14-16H2,1-2H3\/b29-19+
InChIKey : OKSPDBSTEZCWNM-VUTHCHCSSA-N
Other name(s) : GM113 || CHEMBL2181432
MW : 475.6
Formula : C28H33N3O4
CAS_number :
PubChem : 136015314
UniChem : OKSPDBSTEZCWNM-VUTHCHCSSA-N
Target
Structure : No structure
Families : No family
References (3)
| Title : An easy method for the determination of active concentrations of cholinesterase reactivators in blood samples: Application to the efficacy assessment of non quaternary reactivators compared to HI-6 and pralidoxime in VX-poisoned mice - Calas_2017_Chem.Biol.Interact_267_11 |
| Author(s) : Calas AG , Dias J , Rousseau C , Arboleas M , Touvrey-Loiodice M , Mercey G , Jean L , Renard PY , Nachon F |
| Ref : Chemico-Biological Interactions , 267 :11 , 2017 |
| Abstract : |
| PubMedSearch : Calas_2017_Chem.Biol.Interact_267_11 |
| PubMedID: 26972668 |
| Title : Phenyltetrahydroisoquinoline-pyridinaldoxime conjugates as efficient uncharged reactivators for the dephosphylation of inhibited human acetylcholinesterase - Mercey_2012_J.Med.Chem_55_10791 |
| Author(s) : Mercey G , Renou J , Verdelet T , Kliachyna M , Baati R , Gillon E , Arboleas M , Loiodice M , Nachon F , Jean L , Renard PY |
| Ref : Journal of Medicinal Chemistry , 55 :10791 , 2012 |
| Abstract : |
| PubMedSearch : Mercey_2012_J.Med.Chem_55_10791 |
| PubMedID: 23148598 |
| Title : First efficient uncharged reactivators for the dephosphylation of poisoned human acetylcholinesterase - Mercey_2011_Chem.Commun.(Camb)_47_5295 |
| Author(s) : Mercey G , Verdelet T , Saint-Andre G , Gillon E , Wagner A , Baati R , Jean L , Nachon F , Renard PY |
| Ref : Chem Commun (Camb) , 47 :5295 , 2011 |
| Abstract : |
| PubMedSearch : Mercey_2011_Chem.Commun.(Camb)_47_5295 |
| PubMedID: 21451868 |