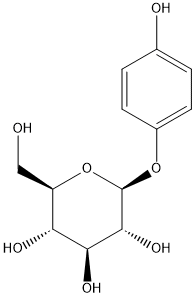
Arbutin
arbutin derivatives such as feruloyl arbutin and caffeoyl arbutin exhibit antioxidative or UV-absorbing activities || Extracted from the dried leaves of bearberry plant in the genus Arctostaphylos and other plants commonly in the Ericaceae family, arbutin is a beta-D-glucopyranoside of [DB09526]. It is found in foods, over-the-counter drugs, and herbal dietary supplements. Most commonly, it is an active ingredient in skincare and cosmetic products as a skin-lightening agent for the prevention of melanin formation in various skin conditions that involve cutaneous hyperpigmentation or hyperactive melanocyte function. It has also been used as an anti-infective for the urinary system as well as a diuretic. Arbutin is available in both natural and synthetic forms. Arbutin is a competitive inhibitor of tyrosinase (E.C.1.14.18.1) in melanocytes, and the inhibition of melanin synthesis at non-toxic concentrations was observed in vitro
General
Type : Natural, Antioxidant
Chemical_Nomenclature : (2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-(4-hydroxyphenoxy)oxane-3,4,5-triol
Canonical SMILES : C1=CC(=CC=C1O)OC2C(C(C(C(O2)CO)O)O)O
InChI : InChI=1S\/C12H16O7\/c13-5-8-9(15)10(16)11(17)12(19-8)18-7-3-1-6(14)2-4-7\/h1-4,8-17H,5H2\/t8-,9-,10+,11-,12-\/m1\/s1
InChIKey : BJRNKVDFDLYUGJ-RMPHRYRLSA-N
Other name(s) : Arbutoside || Ursin || Uvasol || CHEBI:18305 || CHEMBL232202
MW : 272.25
Formula : C12H16O7
CAS_number : 497-76-7
PubChem : 440936
UniChem : BJRNKVDFDLYUGJ-RMPHRYRLSA-N
Target
Structures : No structure
Families : Lipase_3
References (33)
| Title : Immobilization of Thermomyces lanuginosus lipase in a novel polysaccharide-based hydrogel by a two-step crosslinking method and its use in the lauroylation of alpha-arbutin - Chen_2024_Bioresour.Bioprocess_11_7 |
| Author(s) : Chen M , She W , Zhao X , Chen C , Zhu B , Sun Y , Yao Z |
| Ref : Bioresour Bioprocess , 11 :7 , 2024 |
| Abstract : |
| PubMedSearch : Chen_2024_Bioresour.Bioprocess_11_7 |
| PubMedID: 38647918 |
| Title : Immobilization of Thermomyces lanuginosus lipase in a novel polysaccharide-based hydrogel by a two-step crosslinking method and its use in the lauroylation of alpha-arbutin - Chen_2024_Bioresour.Bioprocess_11_7 |
| Author(s) : Chen M , She W , Zhao X , Chen C , Zhu B , Sun Y , Yao Z |
| Ref : Bioresour Bioprocess , 11 :7 , 2024 |
| Abstract : |
| PubMedSearch : Chen_2024_Bioresour.Bioprocess_11_7 |
| PubMedID: 38647918 |
| Title : Immobilization of Thermomyces lanuginosus lipase in a novel polysaccharide-based hydrogel by a two-step crosslinking method and its use in the lauroylation of alpha-arbutin - Chen_2024_Bioresour.Bioprocess_11_7 |
| Author(s) : Chen M , She W , Zhao X , Chen C , Zhu B , Sun Y , Yao Z |
| Ref : Bioresour Bioprocess , 11 :7 , 2024 |
| Abstract : |
| PubMedSearch : Chen_2024_Bioresour.Bioprocess_11_7 |
| PubMedID: 38647918 |
| Title : Arbutin protects brain against middle cerebral artery occlusion-reperfusion (MCAo\/R) injury - Kumar_2021_Biochem.Biophys.Res.Commun_577_52 |
| Author(s) : Kumar M , Singh G , Kushwah AS , Surampalli G , Singh TG , Gupta S |
| Ref : Biochemical & Biophysical Research Communications , 577 :52 , 2021 |
| Abstract : |
| PubMedSearch : Kumar_2021_Biochem.Biophys.Res.Commun_577_52 |
| PubMedID: 34507065 |
| Title : Arbutin attenuates monosodium L-glutamate induced neurotoxicity and cognitive dysfunction in rats - Kumar_2021_Neurochem.Int_151_105217 |
| Author(s) : Kumar M , Kumar A , Sindhu RK , Kushwah AS |
| Ref : Neurochem Int , 151 :105217 , 2021 |
| Abstract : |
| PubMedSearch : Kumar_2021_Neurochem.Int_151_105217 |
| PubMedID: 34710534 |
| Title : Efficient Enzymatic Synthesis of Lipophilic Phenolic Glycoside Azelaic Acid Esters and Their Depigmenting Activity - Xu_2021_J.Agric.Food.Chem_69_13102 |
| Author(s) : Xu H , Li X , Xin X , Mo L , Zou Y , Zhao G |
| Ref : Journal of Agricultural and Food Chemistry , 69 :13102 , 2021 |
| Abstract : |
| PubMedSearch : Xu_2021_J.Agric.Food.Chem_69_13102 |
| PubMedID: 34705451 |
| Title : Synthesis of lipophilic arbutin ester by enzymatic transesterification in high pressure carbon dioxide - Liu_2021_Enzyme.Microb.Technol_148_109818 |
| Author(s) : Liu KJ |
| Ref : Enzyme Microb Technol , 148 :109818 , 2021 |
| Abstract : |
| PubMedSearch : Liu_2021_Enzyme.Microb.Technol_148_109818 |
| PubMedID: 34116761 |
| Gene_locus related to this paper: canar-LipB |
| Title : Synthesis of lipophilic arbutin ester by enzymatic transesterification in high pressure carbon dioxide - Liu_2021_Enzyme.Microb.Technol_148_109818 |
| Author(s) : Liu KJ |
| Ref : Enzyme Microb Technol , 148 :109818 , 2021 |
| Abstract : |
| PubMedSearch : Liu_2021_Enzyme.Microb.Technol_148_109818 |
| PubMedID: 34116761 |
| Gene_locus related to this paper: canar-LipB |
| Title : Synthesis of lipophilic arbutin ester by enzymatic transesterification in high pressure carbon dioxide - Liu_2021_Enzyme.Microb.Technol_148_109818 |
| Author(s) : Liu KJ |
| Ref : Enzyme Microb Technol , 148 :109818 , 2021 |
| Abstract : |
| PubMedSearch : Liu_2021_Enzyme.Microb.Technol_148_109818 |
| PubMedID: 34116761 |
| Gene_locus related to this paper: canar-LipB |
| Title : Arbutin protects brain against middle cerebral artery occlusion-reperfusion (MCAo\/R) injury - Kumar_2021_Biochem.Biophys.Res.Commun_577_52 |
| Author(s) : Kumar M , Singh G , Kushwah AS , Surampalli G , Singh TG , Gupta S |
| Ref : Biochemical & Biophysical Research Communications , 577 :52 , 2021 |
| Abstract : |
| PubMedSearch : Kumar_2021_Biochem.Biophys.Res.Commun_577_52 |
| PubMedID: 34507065 |
| Title : Arbutin attenuates monosodium L-glutamate induced neurotoxicity and cognitive dysfunction in rats - Kumar_2021_Neurochem.Int_151_105217 |
| Author(s) : Kumar M , Kumar A , Sindhu RK , Kushwah AS |
| Ref : Neurochem Int , 151 :105217 , 2021 |
| Abstract : |
| PubMedSearch : Kumar_2021_Neurochem.Int_151_105217 |
| PubMedID: 34710534 |
| Title : Efficient Enzymatic Synthesis of Lipophilic Phenolic Glycoside Azelaic Acid Esters and Their Depigmenting Activity - Xu_2021_J.Agric.Food.Chem_69_13102 |
| Author(s) : Xu H , Li X , Xin X , Mo L , Zou Y , Zhao G |
| Ref : Journal of Agricultural and Food Chemistry , 69 :13102 , 2021 |
| Abstract : |
| PubMedSearch : Xu_2021_J.Agric.Food.Chem_69_13102 |
| PubMedID: 34705451 |
| Title : Arbutin protects brain against middle cerebral artery occlusion-reperfusion (MCAo\/R) injury - Kumar_2021_Biochem.Biophys.Res.Commun_577_52 |
| Author(s) : Kumar M , Singh G , Kushwah AS , Surampalli G , Singh TG , Gupta S |
| Ref : Biochemical & Biophysical Research Communications , 577 :52 , 2021 |
| Abstract : |
| PubMedSearch : Kumar_2021_Biochem.Biophys.Res.Commun_577_52 |
| PubMedID: 34507065 |
| Title : Arbutin attenuates monosodium L-glutamate induced neurotoxicity and cognitive dysfunction in rats - Kumar_2021_Neurochem.Int_151_105217 |
| Author(s) : Kumar M , Kumar A , Sindhu RK , Kushwah AS |
| Ref : Neurochem Int , 151 :105217 , 2021 |
| Abstract : |
| PubMedSearch : Kumar_2021_Neurochem.Int_151_105217 |
| PubMedID: 34710534 |
| Title : Efficient Enzymatic Synthesis of Lipophilic Phenolic Glycoside Azelaic Acid Esters and Their Depigmenting Activity - Xu_2021_J.Agric.Food.Chem_69_13102 |
| Author(s) : Xu H , Li X , Xin X , Mo L , Zou Y , Zhao G |
| Ref : Journal of Agricultural and Food Chemistry , 69 :13102 , 2021 |
| Abstract : |
| PubMedSearch : Xu_2021_J.Agric.Food.Chem_69_13102 |
| PubMedID: 34705451 |
| Title : Significantly Enhanced Synthesis of Aromatic Esters of Arbutin Catalyzed by Immobilized Lipase in Co-solvent Systems - Yang_2020_Front.Bioeng.Biotechnol_8_273 |
| Author(s) : Yang R , Nie Z , Xu N , Zhao X , Wang Z , Luo H |
| Ref : Front Bioeng Biotechnol , 8 :273 , 2020 |
| Abstract : |
| PubMedSearch : Yang_2020_Front.Bioeng.Biotechnol_8_273 |
| PubMedID: 32363180 |
| Title : Significantly Enhanced Synthesis of Aromatic Esters of Arbutin Catalyzed by Immobilized Lipase in Co-solvent Systems - Yang_2020_Front.Bioeng.Biotechnol_8_273 |
| Author(s) : Yang R , Nie Z , Xu N , Zhao X , Wang Z , Luo H |
| Ref : Front Bioeng Biotechnol , 8 :273 , 2020 |
| Abstract : |
| PubMedSearch : Yang_2020_Front.Bioeng.Biotechnol_8_273 |
| PubMedID: 32363180 |
| Title : Significantly Enhanced Synthesis of Aromatic Esters of Arbutin Catalyzed by Immobilized Lipase in Co-solvent Systems - Yang_2020_Front.Bioeng.Biotechnol_8_273 |
| Author(s) : Yang R , Nie Z , Xu N , Zhao X , Wang Z , Luo H |
| Ref : Front Bioeng Biotechnol , 8 :273 , 2020 |
| Abstract : |
| PubMedSearch : Yang_2020_Front.Bioeng.Biotechnol_8_273 |
| PubMedID: 32363180 |
| Title : Novel and highly efficient regioselective route to helicid esters by lipozyme TLL - Yang_2013_PLoS.One_8_e80715 |
| Author(s) : Yang R , Zhao X , Liu X |
| Ref : PLoS ONE , 8 :e80715 , 2013 |
| Abstract : |
| PubMedSearch : Yang_2013_PLoS.One_8_e80715 |
| PubMedID: 24278310 |
| Gene_locus related to this paper: humla-1lipa |
| Title : Novel and highly efficient regioselective route to helicid esters by lipozyme TLL - Yang_2013_PLoS.One_8_e80715 |
| Author(s) : Yang R , Zhao X , Liu X |
| Ref : PLoS ONE , 8 :e80715 , 2013 |
| Abstract : |
| PubMedSearch : Yang_2013_PLoS.One_8_e80715 |
| PubMedID: 24278310 |
| Gene_locus related to this paper: humla-1lipa |
| Title : Novel and highly efficient regioselective route to helicid esters by lipozyme TLL - Yang_2013_PLoS.One_8_e80715 |
| Author(s) : Yang R , Zhao X , Liu X |
| Ref : PLoS ONE , 8 :e80715 , 2013 |
| Abstract : |
| PubMedSearch : Yang_2013_PLoS.One_8_e80715 |
| PubMedID: 24278310 |
| Gene_locus related to this paper: humla-1lipa |
| Title : A highly regioselective route to arbutin esters by immobilized lipase from Penicillium expansum - Yang_2010_Bioresour.Technol_101_1 |
| Author(s) : Yang RL , Li N , Li RF , Smith TJ , Zong MH |
| Ref : Bioresour Technol , 101 :1 , 2010 |
| Abstract : |
| PubMedSearch : Yang_2010_Bioresour.Technol_101_1 |
| PubMedID: 19695875 |
| Title : A highly regioselective route to arbutin esters by immobilized lipase from Penicillium expansum - Yang_2010_Bioresour.Technol_101_1 |
| Author(s) : Yang RL , Li N , Li RF , Smith TJ , Zong MH |
| Ref : Bioresour Technol , 101 :1 , 2010 |
| Abstract : |
| PubMedSearch : Yang_2010_Bioresour.Technol_101_1 |
| PubMedID: 19695875 |
| Title : A highly regioselective route to arbutin esters by immobilized lipase from Penicillium expansum - Yang_2010_Bioresour.Technol_101_1 |
| Author(s) : Yang RL , Li N , Li RF , Smith TJ , Zong MH |
| Ref : Bioresour Technol , 101 :1 , 2010 |
| Abstract : |
| PubMedSearch : Yang_2010_Bioresour.Technol_101_1 |
| PubMedID: 19695875 |
| Title : Enzymatic preparation of arbutin derivatives: lipase-catalyzed direct acylation without the need of vinyl ester as an acyl donor - Ishihara_2010_J.Biosci.Bioeng_109_554 |
| Author(s) : Ishihara K , Katsube Y , Kumazawa N , Kuratani M , Masuoka N , Nakajima N |
| Ref : J Biosci Bioeng , 109 :554 , 2010 |
| Abstract : |
| PubMedSearch : Ishihara_2010_J.Biosci.Bioeng_109_554 |
| PubMedID: 20471593 |
| Gene_locus related to this paper: canar-LipB |
| Title : Enzymatic preparation of arbutin derivatives: lipase-catalyzed direct acylation without the need of vinyl ester as an acyl donor - Ishihara_2010_J.Biosci.Bioeng_109_554 |
| Author(s) : Ishihara K , Katsube Y , Kumazawa N , Kuratani M , Masuoka N , Nakajima N |
| Ref : J Biosci Bioeng , 109 :554 , 2010 |
| Abstract : |
| PubMedSearch : Ishihara_2010_J.Biosci.Bioeng_109_554 |
| PubMedID: 20471593 |
| Gene_locus related to this paper: canar-LipB |
| Title : Enzymatic preparation of arbutin derivatives: lipase-catalyzed direct acylation without the need of vinyl ester as an acyl donor - Ishihara_2010_J.Biosci.Bioeng_109_554 |
| Author(s) : Ishihara K , Katsube Y , Kumazawa N , Kuratani M , Masuoka N , Nakajima N |
| Ref : J Biosci Bioeng , 109 :554 , 2010 |
| Abstract : |
| PubMedSearch : Ishihara_2010_J.Biosci.Bioeng_109_554 |
| PubMedID: 20471593 |
| Gene_locus related to this paper: canar-LipB |
| Title : Synthesis of acyl arbutin by an immobilized lipase and its suppressive ability against lipid oxidation in a bulk system and O\/W emulsion - Nagai_2009_Biosci.Biotechnol.Biochem_73_2501 |
| Author(s) : Nagai M , Watanabe Y , Nomura M |
| Ref : Biosci Biotechnol Biochem , 73 :2501 , 2009 |
| Abstract : |
| PubMedSearch : Nagai_2009_Biosci.Biotechnol.Biochem_73_2501 |
| PubMedID: 19897895 |
| Gene_locus related to this paper: canar-LipB |
| Title : Synthesis of acyl arbutin by an immobilized lipase and its suppressive ability against lipid oxidation in a bulk system and O\/W emulsion - Nagai_2009_Biosci.Biotechnol.Biochem_73_2501 |
| Author(s) : Nagai M , Watanabe Y , Nomura M |
| Ref : Biosci Biotechnol Biochem , 73 :2501 , 2009 |
| Abstract : |
| PubMedSearch : Nagai_2009_Biosci.Biotechnol.Biochem_73_2501 |
| PubMedID: 19897895 |
| Gene_locus related to this paper: canar-LipB |
| Title : Synthesis of acyl arbutin by an immobilized lipase and its suppressive ability against lipid oxidation in a bulk system and O\/W emulsion - Nagai_2009_Biosci.Biotechnol.Biochem_73_2501 |
| Author(s) : Nagai M , Watanabe Y , Nomura M |
| Ref : Biosci Biotechnol Biochem , 73 :2501 , 2009 |
| Abstract : |
| PubMedSearch : Nagai_2009_Biosci.Biotechnol.Biochem_73_2501 |
| PubMedID: 19897895 |
| Gene_locus related to this paper: canar-LipB |
| Title : Lipase-catalyzed synthesis of arbutin cinnamate in an organic solvent and application of transesterification to stabilize plant pigments - Nakajima_1997_Biosci.Biotechnol.Biochem_61_1926 |
| Author(s) : Nakajima N , Ishihara K , Matsumura S , Hamada H , Nakamura K , Furuya T |
| Ref : Biosci Biotechnol Biochem , 61 :1926 , 1997 |
| Abstract : |
| PubMedSearch : Nakajima_1997_Biosci.Biotechnol.Biochem_61_1926 |
| PubMedID: 9404074 |
| Gene_locus related to this paper: burce-lipaa |
| Title : Lipase-catalyzed synthesis of arbutin cinnamate in an organic solvent and application of transesterification to stabilize plant pigments - Nakajima_1997_Biosci.Biotechnol.Biochem_61_1926 |
| Author(s) : Nakajima N , Ishihara K , Matsumura S , Hamada H , Nakamura K , Furuya T |
| Ref : Biosci Biotechnol Biochem , 61 :1926 , 1997 |
| Abstract : |
| PubMedSearch : Nakajima_1997_Biosci.Biotechnol.Biochem_61_1926 |
| PubMedID: 9404074 |
| Gene_locus related to this paper: burce-lipaa |
| Title : Lipase-catalyzed synthesis of arbutin cinnamate in an organic solvent and application of transesterification to stabilize plant pigments - Nakajima_1997_Biosci.Biotechnol.Biochem_61_1926 |
| Author(s) : Nakajima N , Ishihara K , Matsumura S , Hamada H , Nakamura K , Furuya T |
| Ref : Biosci Biotechnol Biochem , 61 :1926 , 1997 |
| Abstract : |
| PubMedSearch : Nakajima_1997_Biosci.Biotechnol.Biochem_61_1926 |
| PubMedID: 9404074 |
| Gene_locus related to this paper: burce-lipaa |