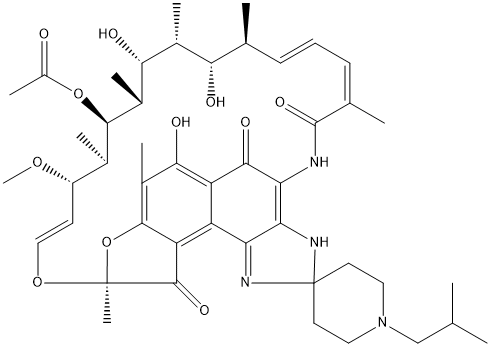
Rifabutin
Rifamycin derivative. A broad-spectrum antibiotic that is being used as prophylaxis. Rifabutin is an antibacterial prescription medicine approved by the U.S. Food and Drug Administration (FDA) for the prevention of disseminated Mycobacterium avium complex (MAC) infection in people with advanced HIV infection. CYP3A/CYP3A4 substrate
General
Type : Pro-Drug, Drug, Not AlphaBeta Hydrolase target, Piperidine, Antibiotic
Chemical_Nomenclature : [(7S,9E,11S,12R,13S,14R,15R,16R,17S,18S,19E,21Z)-2,15,17,32-tetrahydroxy-11-methoxy-3,7,12,14,16,18,22-heptamethyl-1'-(2-methylpropyl)-6,23-dioxospiro[8,33-dioxa-24,27,29-triazapentacyclo[23.6.1.14,7.05,31.026,30]tritriaconta-1(32),2,4,9,19,21,24,26,30-nonaene-28,4'-piperidine]-13-yl] acetate
Canonical SMILES : CC1C=CC=C(C(=O)N=C2C(=C3C(=C4C2=NC5(N4)CCN(CC5)CC(C)C)C6=C(C(=C3O)C)OC(C6=O)(OC=CC(C(C(C(C(C(C1O)C)O)C)OC(=O)C)C)OC)C)O)C
InChI : InChI=1S\/C46H62N4O11\/c1-22(2)21-50-18-16-46(17-19-50)48-34-31-32-39(54)28(8)42-33(31)43(56)45(10,61-42)59-20-15-30(58-11)25(5)41(60-29(9)51)27(7)38(53)26(6)37(52)23(3)13-12-14-24(4)44(57)47-36(40(32)55)35(34)49-46\/h12-15,20,22-23,25-27,30,37-38,41,48,52-55H,16-19,21H2,1-11H3\/b13-12+,20-15+,24-14-,47-36?\/t23-,25+,26+,27+,30-,37-,38+,41+,45-\/m0\/s1
InChIKey : ZWBTYMGEBZUQTK-PVLSIAFMSA-N
Other name(s) : Ansatipin || Mycobutin || Alfacid || Ansatipine || Rifabutine || SCHEMBL36043 || DB00615 || D00424 || CHEBI:45367
Target
Structures : No structure
Families : Arylacetamide_deacetylase
References (9)
| Title : Human arylacetamide deacetylase is responsible for deacetylation of rifamycins: rifampicin, rifabutin, and rifapentine - Nakajima_2011_Biochem.Pharmacol_82_1747 |
| Author(s) : Nakajima A , Fukami T , Kobayashi Y , Watanabe A , Nakajima M , Yokoi T |
| Ref : Biochemical Pharmacology , 82 :1747 , 2011 |
| Abstract : |
| PubMedSearch : Nakajima_2011_Biochem.Pharmacol_82_1747 |
| PubMedID: 21856291 |
| Gene_locus related to this paper: human-AADAC |
| Title : Human arylacetamide deacetylase is responsible for deacetylation of rifamycins: rifampicin, rifabutin, and rifapentine - Nakajima_2011_Biochem.Pharmacol_82_1747 |
| Author(s) : Nakajima A , Fukami T , Kobayashi Y , Watanabe A , Nakajima M , Yokoi T |
| Ref : Biochemical Pharmacology , 82 :1747 , 2011 |
| Abstract : |
| PubMedSearch : Nakajima_2011_Biochem.Pharmacol_82_1747 |
| PubMedID: 21856291 |
| Gene_locus related to this paper: human-AADAC |
| Title : Human arylacetamide deacetylase is responsible for deacetylation of rifamycins: rifampicin, rifabutin, and rifapentine - Nakajima_2011_Biochem.Pharmacol_82_1747 |
| Author(s) : Nakajima A , Fukami T , Kobayashi Y , Watanabe A , Nakajima M , Yokoi T |
| Ref : Biochemical Pharmacology , 82 :1747 , 2011 |
| Abstract : |
| PubMedSearch : Nakajima_2011_Biochem.Pharmacol_82_1747 |
| PubMedID: 21856291 |
| Gene_locus related to this paper: human-AADAC |
| Title : Rifabutin autoinduction is caused by involvement of cytochrome P450 and cholinesterase - Zhaoxu_2008_Pharmazie_63_156 |
| Author(s) : Zhaoxu L , Jingcheng T , Jinnan Z |
| Ref : Pharmazie , 63 :156 , 2008 |
| Abstract : |
| PubMedSearch : Zhaoxu_2008_Pharmazie_63_156 |
| PubMedID: 18380404 |
| Title : Rifabutin autoinduction is caused by involvement of cytochrome P450 and cholinesterase - Zhaoxu_2008_Pharmazie_63_156 |
| Author(s) : Zhaoxu L , Jingcheng T , Jinnan Z |
| Ref : Pharmazie , 63 :156 , 2008 |
| Abstract : |
| PubMedSearch : Zhaoxu_2008_Pharmazie_63_156 |
| PubMedID: 18380404 |
| Title : Rifabutin autoinduction is caused by involvement of cytochrome P450 and cholinesterase - Zhaoxu_2008_Pharmazie_63_156 |
| Author(s) : Zhaoxu L , Jingcheng T , Jinnan Z |
| Ref : Pharmazie , 63 :156 , 2008 |
| Abstract : |
| PubMedSearch : Zhaoxu_2008_Pharmazie_63_156 |
| PubMedID: 18380404 |
| Title : Metabolism of rifabutin in human enterocyte and liver microsomes: kinetic parameters, identification of enzyme systems, and drug interactions with macrolides and antifungal agents - Iatsimirskaia_1997_Clin.Pharmacol.Ther_61_554 |
| Author(s) : Iatsimirskaia E , Tulebaev S , Storozhuk E , Utkin I , Smith D , Gerber N , Koudriakova T |
| Ref : Clinical Pharmacology & Therapeutics , 61 :554 , 1997 |
| Abstract : |
| PubMedSearch : Iatsimirskaia_1997_Clin.Pharmacol.Ther_61_554 |
| PubMedID: 9164417 |
| Title : Metabolism of rifabutin in human enterocyte and liver microsomes: kinetic parameters, identification of enzyme systems, and drug interactions with macrolides and antifungal agents - Iatsimirskaia_1997_Clin.Pharmacol.Ther_61_554 |
| Author(s) : Iatsimirskaia E , Tulebaev S , Storozhuk E , Utkin I , Smith D , Gerber N , Koudriakova T |
| Ref : Clinical Pharmacology & Therapeutics , 61 :554 , 1997 |
| Abstract : |
| PubMedSearch : Iatsimirskaia_1997_Clin.Pharmacol.Ther_61_554 |
| PubMedID: 9164417 |
| Title : Metabolism of rifabutin in human enterocyte and liver microsomes: kinetic parameters, identification of enzyme systems, and drug interactions with macrolides and antifungal agents - Iatsimirskaia_1997_Clin.Pharmacol.Ther_61_554 |
| Author(s) : Iatsimirskaia E , Tulebaev S , Storozhuk E , Utkin I , Smith D , Gerber N , Koudriakova T |
| Ref : Clinical Pharmacology & Therapeutics , 61 :554 , 1997 |
| Abstract : |
| PubMedSearch : Iatsimirskaia_1997_Clin.Pharmacol.Ther_61_554 |
| PubMedID: 9164417 |