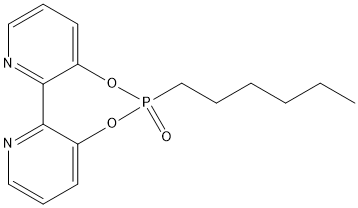
6RKY-K7K-phosphodiester
covalent inhibitor used to transform an esterase into an enantioselective catecholase through bioconjugation of this versatile metal-chelating inhibitor. The symmetry of the molecule allows nucleophilic enzymatic hydrolysis that can lead to only one hydrolytic product that retains the bipyridine moiety within the active site, compared to 6RKY-K7K. The compound in PDB is ZK8
General
Type : Multitarget, Organophosphate, Pyridine, Metal ion chelator
Chemical_Nomenclature : 6-hexyl-[1,3,2]dioxaphosphepino[5,4-b:6,7-b']dipyridine 6-oxide
Canonical SMILES : C13=CC=CN=C1C2=NC=CC=C2O[P](CCCCCC)(=O)O3
InChI : InChI=1S\/C16H19N2O3P\/c1-2-3-4-5-12-22(19)20-13-8-6-10-17-15(13)16-14(21-22)9-7-11-18-16\/h6-11H,2-5,12H2,1H3
InChIKey : XGLVQRVNSLHFKZ-UHFFFAOYSA-N
Other name(s) : ZK8 precursor
Target
Families : 6RKY-K7K-phosphodiester ligand of proteins in family
Hormone-sensitive_lipase_like
Protein :
9zzzz-a0a2k8jn75
References (1)
| Title : Transforming an esterase into an enantioselective catecholase through bioconjugation of a versatile metal-chelating inhibitor - Fernandez-Lopez_2023_Chem.Commun.(Camb)__ |
| Author(s) : Fernandez-Lopez L , Cea-Rama I , Alvarez-Malmagro J , Ressmann AK , Gonzalez-Alfonso JL , Coscolin C , Shahgaldian P , Plou FJ , Modregger J , Pita M , Sanz-Aparicio J , Ferrer M |
| Ref : Chem Commun (Camb) , : , 2023 |
| Abstract : |
| PubMedSearch : Fernandez-Lopez_2023_Chem.Commun.(Camb)__ |
| PubMedID: 37376994 |
| Gene_locus related to this paper: 9zzzz-a0a2k8jn75 |